IXIARO Japanese Encephalitis Vaccine Ref.[10105] Active ingredients: Japanese encephalitis, live attenuated
Source: FDA, National Drug Code (US) Revision Year: 2018
1. Indications and Usage
IXIARO is a vaccine indicated for the prevention of disease caused by Japanese encephalitis virus (JEV). IXIARO is approved for use in individuals 2 months of age and older.
2. Dosage and Administration
For intramuscular administration only.
2.1 Dosage and Schedule
Primary Series
Children 2 months to <3 years of age: Primary immunization with IXIARO consists of two (2) 0.25 mL doses, administered 28 days apart.
Individuals 3 years of age and older: Primary immunization with IXIARO consists of two (2) 0.5 mL doses, administered 28 days apart.
Complete the primary immunization series at least 1 week prior to potential exposure to JEV.
Booster Dose
Individuals 17 years of age and older: If the primary series of two doses was completed more than 1 year previously, a booster dose may be given if ongoing exposure or re-exposure to JEV is expected.
Infants, children and adolescents 2 months to <17 years of age: The safety and immunogenicity of a booster dose has not been evaluated.
2.2 Administration
IXIARO is administered intramuscularly. The preferred sites for intramuscular injection are the anterolateral aspect of the thigh in infants 2 to 11 months of age, the anterolateral aspect of the thigh (or the deltoid muscle if muscle mass is adequate) in children 1 to <3 years of age, or the deltoid muscle in individuals 3 years of age and older.
Do not administer intravenously, intradermally, or subcutaneously.
2.3 Preparation for Administration
Prior to agitation, IXIARO is a clear liquid with a white precipitate. Before administration, shake the syringe well to obtain a white, opaque, homogeneous suspension.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration. If either of these conditions exists, do not administer.
Preparation of a 0.25 mL Dose of IXIARO for Administration to Children 2 Months to <3 Years of Age:
- Shake the pre-filled syringe containing 0.5 mL to obtain a homogeneous suspension.
- Remove the syringe tip cap by gently twisting it. Do not attempt to snap or pull the tip off as this may damage the syringe.
- Attach a sterile safety needle to the pre-filled syringe (needle is not provided with IXIARO).
- Hold the syringe in an upright position and uncap the needle.
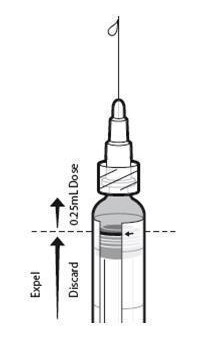
- Push the plunger stopper up to the edge of the red line on the syringe barrel, indicated by a red arrow (see Figure1)*, and discard expelled volume into a medical waste container.
- Lock the needle safety shield and remove the needle.
- Attach a new sterile needle prior to injection of the remaining volume.
* If the plunger stopper is pushed beyond the red line, do not administer the vaccine. Repeat the procedure using a new pre-filled syringe.
Figure 1. Preparation for Administration of 0.25 mL:
Preparation of a 0.5 mL Dose of IXIARO for Administration to Individuals 3 Years of Age and Above:
- Shake the pre-filled syringe containing 0.5 mL to obtain a homogeneous suspension.
- Remove the syringe tip cap by gently twisting it. Do not attempt to snap or pull the tip off as this may damage the syringe.
- Attach a sterile needle to the pre-filled syringe (needle is not provided with IXIARO).
16.2. Storage and Handling
Store in a refrigerator at 2° to 8°C (35° to 46°F). Do not freeze.
Do not use the vaccine after the expiration date shown on the label. Store in the original package in order to protect from light. During storage, a clear liquid with a white precipitate can be observed.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.