JCOVDEN Suspension for injection Ref.[11085] Active ingredients: SARS-CoV-2 Spike glycoprotein (Ad26.COV2-S)
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Belgium
4.1. Therapeutic indications
JCOVDEN is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 in individuals 18 years of age and older.
The use of this vaccine should be in accordance with official recommendations.
4.2. Posology and method of administration
Posology
Individuals 18 years of age and older
Primary vaccination
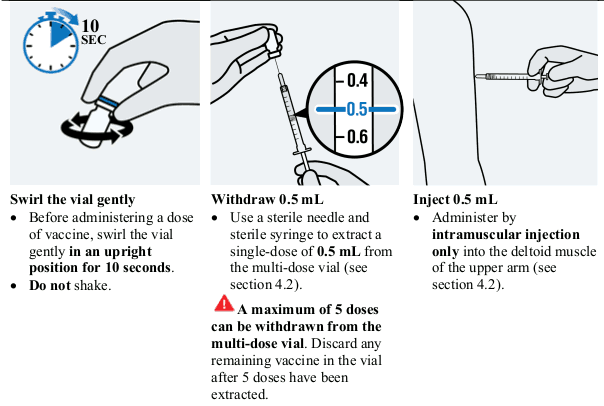
JCOVDEN is administered as a single-dose of 0.5 mL by intramuscular injection only.
Booster dose
A booster dose (second dose) of 0.5 mL of JCOVDEN may be administered intramuscularly at least 2 months after the primary vaccination in individuals 18 years of age and older (see also sections 4.4, 4.8 and 5.1).
A booster dose of the JCOVDEN (0.5 mL) may be administered as a heterologous booster dose following completion of primary vaccination with an approved mRNA COVID-19 vaccine. The dosing interval for the heterologous booster dose is the same as that authorised for a booster dose of the vaccine used for primary vaccination (see also sections 4.4, 4.8 and 5.1).
Paediatric population
The safety and efficacy of JCOVDEN in children and adolescents (less than 18 years of age) have not yet been established. No data are available.
Elderly
No dose adjustment is required in elderly individuals ≥ 65 years of age. See also sections 4.8 and 5.1.
Method of administration
JCOVDEN is for intramuscular injection only, preferably in the deltoid muscle of the upper arm.
Do not inject the vaccine intravascularly, intravenously, subcutaneously or intradermally.
The vaccine should not be mixed in the same syringe with any other vaccines or medicinal products.
For precautions to be taken before administering the vaccine, see section 4.4.
For instructions on handling and disposal of the vaccine, see section 6.6.
4.9. Overdose
No case of overdose has been reported. In phase ½ studies where a higher dose (up to 2-fold) was administered JCOVDEN remained well-tolerated, however vaccinated individuals reported an increase in reactogenicity (increased vaccination site pain, fatigue, headache, myalgia, nausea and pyrexia).
In the event of overdose, monitoring of vital functions and possible symptomatic treatment is recommended.
6.3. Shelf life
Unopened vial
2 years when stored at -25°C to -15°C.
Once removed from the freezer, the unopened vaccine may be stored refrigerated at 2°C to 8°C, protected from light, for a single period of up to 3 months, not exceeding the printed expiry date (EXP).
Once thawed, the vaccine should not be re-frozen.
For special precautions for storage, see section 6.4.
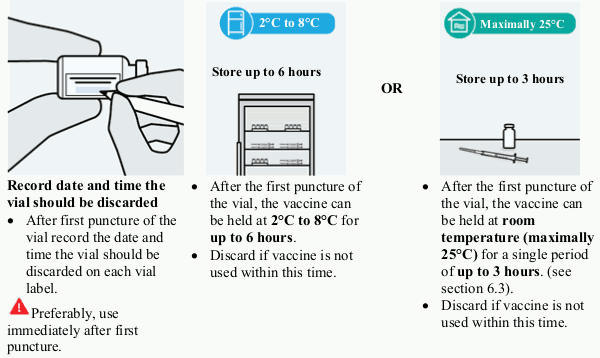
Opened vial (after first puncture of the vial)
Chemical and physical in-use stability of the vaccine has been demonstrated for 6 hours at 2°C to 25°C. From a microbiological point of view, the product should preferably be used immediately after first puncture of the vial; however, the product can be stored between 2°C-8°C for a maximum of 6 hours or remain at room temperature (maximally 25°C) up to 3 hours after first puncture of the vial. Beyond these times, in-use storage is the responsibility of the user.
6.4. Special precautions for storage
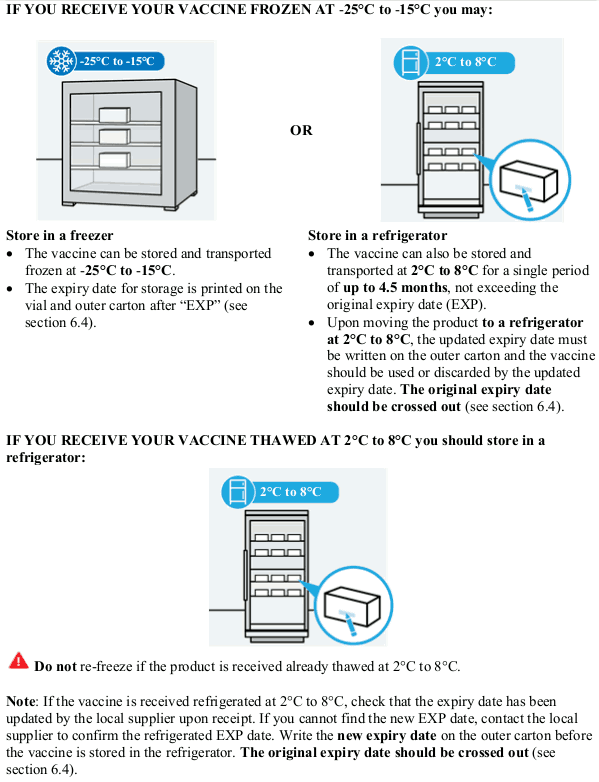
Store and transport frozen at -25°C to -15°C. The expiry date for storage at -25°C to -15°C is printed on the vial and outer carton after "EXP".
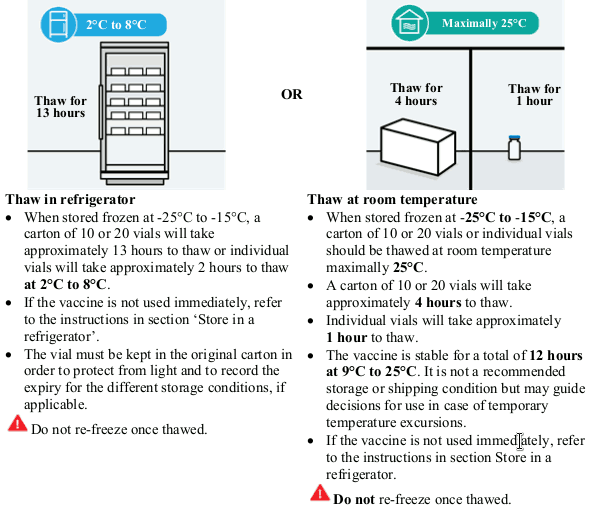
When stored frozen at -25°C to -15°C, the vaccine can be thawed either at 2°C to 8°C or at room temperature:
- at 2°C to 8°C: a carton of 10 or 20 vials will take approximately 13 hours to thaw, and a single vial will take approximately 2 hours to thaw.
- at room temperature (maximally 25°C): a carton of 10 or 20 vials will take approximately 4 hours to thaw, and a single vial will take approximately 1 hour to thaw.
The vaccine can also be stored in a refrigerator or transported at 2°C to 8°C for a single period of up to 4.5 months, not exceeding the original expiry date (EXP). Upon moving the product to 2°C to 8°C storage, the updated expiry date must be written on the outer carton and the vaccine should be used or discarded by the updated expiry date. The original expiry date should be crossed out. The vaccine can also be transported at 2°C to 8°C as long as the appropriate storage conditions (temperature, time) are applied.
Once thawed, the vaccine cannot be re-frozen.
Keep the vials in the original carton in order to protect from light.
Unopened JCOVDEN is stable for a total of 12 hours at 9°C to 25°C. It is not a recommended storage or shipping condition but may guide decisions for use in case of temporary temperature excursions during the 4.5 month storage at 2°C to 8°C.
For storage conditions after first opening of the medicinal product, see section 6.3.
6.5. Nature and contents of container
A 2.5 mL suspension in a multi-dose vial (type I glass) with a rubber stopper (chlorobutyl with fluoropolymer coated surface), aluminium crimp and blue plastic cap. Each vial contains 5 doses of 0.5 mL.
Pack sizes of 10 or 20 multi-dose vials.
Not all pack sizes may be marketed.
6.6. Special precautions for disposal and other handling
Handling instructions and administration
This vaccine should be handled by a healthcare professional using aseptic technique to ensure the sterility of each dose.
- The vaccine comes ready to use once thawed.
- The vaccine may be supplied frozen at -25°C to -15°C or thawed at 2°C to 8°C.
- Do not re-freeze vaccine once thawed.
- Keep the vials in the original carton in order to protect from light and to record the expiry for the different storage conditions, if applicable.
a. Storage upon receipt of vaccine
b. If stored frozen, thaw vial(s) either in a refrigerator or at room temperature before administration
c. Inspect vial and vaccine
- JCOVDEN is a colorless to slightly yellow, clear to very opalescent suspension (pH 6-6.4).
- The vaccine should be inspected visually for particulate matter and discoloration prior to administration.
- The vial should be inspected visually for cracks or any abnormalities, such as evidence of tampering prior to administration.
If any of these should exist, do not administer the vaccine.
d. Prepare and administer vaccine
e. Storage after first puncture
f. Disposal
Any unused vaccine or waste material should be disposed of in compliance with local guidance for pharmaceutical waste. Potential spills should be disinfected with agents with viricidal activity against adenovirus.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.