JEVTANA Concentrate and solvent for solution for infusion Ref.[6469] Active ingredients: Cabazitaxel
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Sanofi-aventis groupe, 54, rue La Boétie, F-75008 Paris, France
Therapeutic indications
JEVTANA in combination with prednisone or prednisolone is indicated for the treatment of adult patients with metastatic castration resistant prostate cancer previously treated with a docetaxel-containing regimen (see section 5.1).
Posology and method of administration
The use of JEVTANA should be confined to units specialised in the administration of cytotoxics and it should only be administered under the supervision of a physician experienced in the use of anticancer chemotherapy. Facilities and equipment for the treatment of serious hypersensitivity reactions like hypotension and bronchospasm must be available (see section 4.4).
Premedication
The recommended premedication regimen should be performed at least 30 minutes prior to each administration of JEVTANA with the following intravenous medicinal products to mitigate the risk and severity of hypersensitivity:
- antihistamine (dexchlorpheniramine 5 mg or diphenhydramine 25 mg or equivalent),
- corticosteroid (dexamethasone 8 mg or equivalent), and
- H2 antagonist (ranitidine or equivalent) (see section 4.4).
Antiemetic prophylaxis is recommended and can be given orally or intravenously as needed.
Throughout the treatment, adequate hydration of the patient needs to be ensured, in order to prevent complications like renal failure.
Posology
The recommended dose of JEVTANA is 25 mg/m² administered as a 1 hour intravenous infusion every 3 weeks in combination with oral prednisone or prednisolone 10 mg administered daily throughout treatment.
Dose adjustments
Dose modifications should be made if patients experience the following adverse reactions (Grades refer to Common Terminology Criteria for Adverse Events [CTCAE 4.0]): Table 1 - Recommended dose modifications for adverse reaction in patients treated with cabazitaxel
| Adverse reactions | Dose modification |
|---|---|
| Prolonged grade ≥3 neutropenia (longer than 1 week) despite appropriate treatment including G-CSF | Delay treatment until neutrophil count is >1,500 cells/mm³, then reduce cabazitaxel dose from 25 mg/m² to 20 mg/m². |
| Febrile neutropenia or neutropenic infection | Delay treatment until improvement or resolution, and until neutrophil count is >1,500 cells/mm³, then reduce cabazitaxel dose from 25 mg/m² to 20 mg/m². |
| Grade ≥3 diarrhoea or persisting diarrhoea despite appropriate treatment, including fluid and electrolytes replacement | Delay treatment until improvement or resolution, then reduce cabazitaxel dose from 25 mg/m² to 20 mg/m². |
| Grade >2 peripheral neuropathy | Delay treatment until improvement, then reduce cabazitaxel dose from 25 mg/m² to 20 mg/m². |
If patients continue to experience any of these reactions at 20 mg/m², further dose reduction to 15 mg/m² or discontinuation of JEVTANA may be considered. Data in patients below the 20 mg/m² dose are limited.
Special populations
Patients with hepatic impairment
Cabazitaxel is extensively metabolised by the liver. Patients with mild hepatic impairment (total bilirubin >1 to ≤1.5 x Upper Limit of Normal (ULN) or AST >1.5 x ULN), should have cabazitaxel dose reduced to 20 mg/m². Administration of cabazitaxel to patients with mild hepatic impairment should be undertaken with caution and close monitoring of safety. In patients with moderate hepatic impairment (total bilirubin >1.5 to ≤3.0 x ULN), the maximum tolerated dose (MTD) was 15 mg/m². If the treatment is envisaged in patients with moderate hepatic impairment the dose of cabazitaxel should not exceed 15 mg/m². However, limited efficacy data are available at this dose.
Cabazitaxel should not be given to patients with severe hepatic impairment (total bilirubin >3 x ULN) (see sections 4.3, 4.4 and 5.2).
Patients with renal impairment
Cabazitaxel is minimally excreted through the kidney. No dose adjustment is necessary in patients with renal impairment, not requiring hemodialysis. Patients presenting end stage renal disease (creatinine clearance (CLCR <15 mL/min/1.73 m²), by their condition and the limited amount of data available should be treated with caution and monitored carefully during treatment (see sections 4.4 and 5.2).
Elderly
No specific dose adjustment for the use of cabazitaxel in elderly patients is recommended (see also sections 4.4, 4.8 and 5.2).
Concomitant medicinal products use
Concomitant medicinal products that are strong inducers or strong inhibitors of CYP3A should be avoided. However, if patients require co-administration of a strong CYP3A inhibitor, a 25% cabazitaxel dose reduction should be considered (see sections 4.4 and 4.5).
Paediatric population
There is no relevant use of JEVTANA in the paediatric population. The safety and the efficacy of JEVTANA in children and adolescents below 18 years of age have not been established (see section 5.1).
Method of administration
For instructions on preparation and administration of the product, see section 6.6.
PVC infusion containers and polyurethane infusion sets should not be used.
JEVTANA must not be mixed with any other medicinal products than those mentioned in section 6.6.
Overdose
There is no known antidote to cabazitaxel. The anticipated complications of overdose would consist of exacerbation of adverse reactions as bone marrow suppression and gastrointestinal disorders. In case of overdose, the patient should be kept in a specialised unit and closely monitored. Patients should receive therapeutic G-CSF as soon as possible after discovery of overdose. Other appropriate symptomatic measures should be taken.
Shelf life
Unopened vials: 3 years.
After opening: The concentrate and solvent vials must be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user.
After initial dilution of the concentrate with the solvent: Chemical and physical in-use stability has been demonstrated for 1 hour at ambient temperature (15°C-30°C). From a microbiological point of view, the concentrate-solvent mixture should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user and would normally not be longer than 24 hour at 2°C-8°C, unless dilution has taken place in controlled and validated aseptic conditions.
After final dilution in the infusion bag/bottle: Chemical and physical stability of the infusion solution has been demonstrated for 8 hours at ambient temperature (including the 1-hour infusion time) and for 48 hours at refrigerated conditions (including the 1-hour infusion time). From a microbiological point of view, the infusion solution should be used immediately. If not used immediately, in-use storage times and conditions are the responsibility of the user and would normally not be longer than 24 hour at 2°C-8°C, unless dilution has taken place in controlled and validated aseptic conditions.
Special precautions for storage
Do not store above 30°C.
Do not refrigerate.
For storage conditions after dilution of the medicinal product, see section 6.3.
Nature and contents of container
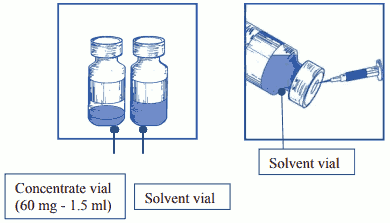
One pack contains one vial of concentrate and one vial of solvent:
Concentrate: 1.5 ml of concentrate in a 15 ml clear glass vial (type I) closed with a grey chlorobutyl rubber closure sealed by an aluminium cap covered with a light green plastic flip-off cap. Each vial contains 60 mg cabazitaxel per 1.5 ml nominal volume (fill volume: 73.2 mg of cabazitaxel/1.83 ml). This fill volume has been established during the development of JEVTANA to compensate for liquid loss during preparation of the premix. This overfill ensures that after dilution with the entire content of the accompanying solvent for JEVTANA, there is a minimal extractable premix volume of 6 ml containing 10 mg/ml JEVTANA which corresponds to the labelled amount of 60 mg per vial.
Solvent: 4.5 ml of solvent in a 15 ml clear glass vial (type I) closed with a grey chlorobutyl rubber closure sealed by a gold colour aluminium cap covered with a colourless plastic flip-off cap. Each vial contains 4.5 ml nominal volume (fill volume: 5.67 ml). This fill volume has been established during the development and the overfill ensures, after the addition of the entire content of the solvent vial to the content of JEVTANA 60 mg concentrate vial, a concentration of the premix solution of 10 mg/ml JEVTANA.
Special precautions for disposal and other handling
JEVTANA should only be prepared and administered by personnel trained in handling cytotoxic agents. Pregnant staff should not handle the product. As for any other antineoplastic agent, caution should be exercised when handling and preparing JEVTANA solutions, taking into account the use of containment devices, personal protective equipment (e.g. gloves), and preparation procedures. If JEVTANA, at any step of its handling, should come into contact with the skin, wash immediately and thoroughly with soap and water. If it should come into contact with mucous membranes, wash immediately and thoroughly with water.
Always dilute the concentrate for solution for infusion with the entire supplied solvent before adding to infusion solution.
Read this ENTIRE section carefully before mixing and diluting. JEVTANA requires TWO dilutions prior to administration. Follow the preparation instructions provided below.
Note: Both the JEVTANA 60 mg/1.5 ml concentrate vial (fill volume: 73.2 mg of cabazitaxel/1.83 ml) and the solvent vial (fill volume: 5.67 ml) contain an overfill to compensate for liquid loss during preparation. This overfill ensures that after dilution with the ENTIRE contents of the accompanying solvent, there is solution containing 10 mg/ml cabazitaxel.
The following two-step dilution process must be carried out in an aseptic manner for preparing the solution for infusion.
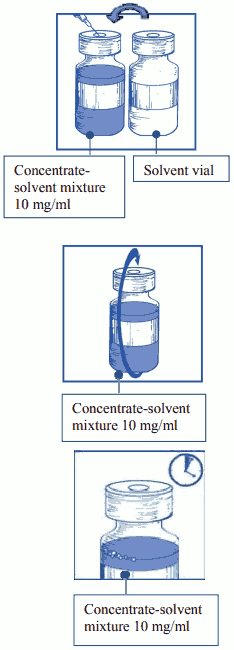
Step 1: Initial dilution of the concentrate for solution for infusion with the supplied solvent
Step 1.1: Inspect the concentrate vial and the supplied solvent. The concentrate solution and the solvent should be clear.
Step 1.2: Using a syringe fitted with a needle, aseptically withdraw the entire contents of the supplied solvent by partially inverting the vial.
Step 1.3: Inject the entire contents into the corresponding concentrate vial. To limit foaming as much as possible when injecting the solvent, direct the needle onto the inside wall of the vial of concentrate solution and inject slowly.
Once reconstituted, the resultant solution contains 10 mg/ml of cabazitaxel.
Step 1.4: Remove the syringe and needle and mix manually and gently by repeated inversions until obtaining a clear and homogeneous solution. It could take approximately 45 seconds.
Step 1.5: Let this solution stand for approximately 5 minutes and check then that the solution is homogeneous and clear. It is normal for foam to persist after this time period.
This resulting concentrate-solvent mixture contains 10 mg/ml of cabazitaxel (at least 6 ml deliverable volume). The second dilution should be done immediately (within 1 hour) as detailed in Step 2.
More than one vial of the concentrate-solvent mixture may be necessary to administer the prescribed dose.
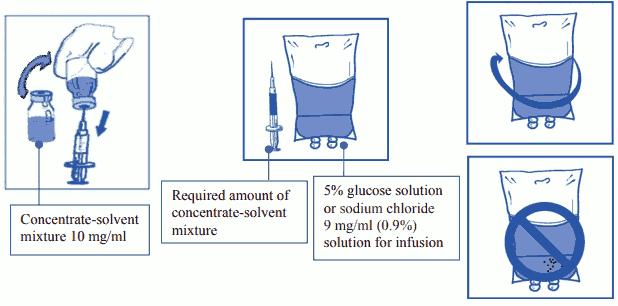
Step 2: Second (final) dilution for infusion
Step 2.1: Aseptically withdraw the required amount of concentrate-solvent mixture (10 mg/ml of cabazitaxel), with a graduated syringe fitted with a needle. As an example, a dose of 45 mg JEVTANA would require 4.5 ml of the concentrate-solvent mixture prepared following Step 1. Since foam may persist on the wall of the vial of this solution, following its preparation described in Step 1, it is preferable to place the needle of the syringe in the middle when extracting.
Step 2.2: Inject in a sterile PVC-free container of either 5% glucose solution or sodium chloride 9 mg/ml (0.9%) solution for infusion. The concentration of the infusion solution should be between 0.10 mg/ml and 0.26 mg/ml.
Step 2.3: Remove the syringe and mix the content of the infusion bag or bottle manually using a rocking motion.
Step 2.4: As with all parenteral products, the resulting infusion solution should be visually inspected prior to use. As the infusion solution is supersaturated, it may crystallize over time. In this case, the solution must not be used and should be discarded.
The infusion solution should be used immediately. However, in-use storage time can be longer under specific conditions mentioned in section 6.3.
An in-line filter of 0.22 micrometer nominal pore size (also referred to as 0.2 micrometer) is recommended during administration.
Do not use PVC infusion containers or polyurethane infusion sets for the preparation and administration of JEVTANA.
JEVTANA must not be mixed with any other medicinal products than those mentioned.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.