JUBBONTI Solution for injection Ref.[109547] Active ingredients: Denosumab
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
Denosumab products bind to RANKL, a transmembrane or soluble protein essential for the formation, function, and survival of osteoclasts, the cells responsible for bone resorption. Denosumab products prevent RANKL from activating its receptor, RANK, on the surface of osteoclasts and their precursors. Prevention of the RANKL/RANK interaction inhibits osteoclast formation, function, and survival, thereby decreasing bone resorption and increasing bone mass and strength in both cortical and trabecular bone.
12.2. Pharmacodynamics
In clinical studies, treatment with 60 mg of denosumab resulted in reduction in the bone resorption marker serum type 1 C-telopeptide (CTX) by approximately 85% by 3 days, with maximal reductions occurring by 1 month. CTX levels were below the limit of assay quantitation (0.049 ng/mL) in 39% to 68% of patients 1 to 3 months after dosing of denosumab. At the end of each dosing interval, CTX reductions were partially attenuated from a maximal reduction of ≥ 87% to ≥ 45% (range: 45% to 80%), as serum denosumab levels diminished, reflecting the reversibility of the effects of denosumab on bone remodeling. These effects were sustained with continued treatment. Upon reinitiation, the degree of inhibition of CTX by denosumab was similar to that observed in patients initiating denosumab treatment.
Consistent with the physiological coupling of bone formation and resorption in skeletal remodeling, subsequent reductions in bone formation markers (i.e., osteocalcin and procollagen type 1 N-terminal peptide [P1NP]) were observed starting 1 month after the first dose of denosumab. After discontinuation of denosumab therapy, markers of bone resorption increased to levels 40% to 60% above pretreatment values but returned to baseline levels within 12 months.
12.3. Pharmacokinetics
In a study conducted in healthy male and female volunteers (n = 73, age range: 18 to 64 years) following a single subcutaneously administered denosumab dose of 60 mg after fasting (at least for 12 hours), the mean maximum denosumab concentration (Cmax) was 6.75 mcg/mL (standard deviation [SD] = 1.89 mcg/mL). The median time to maximum denosumab concentration (Tmax) was 10 days (range: 3 to 21 days). After Cmax, serum denosumab concentrations declined over a period of 4 to 5 months with a mean half-life of 25.4 days (SD = 8.5 days; n = 46). The mean area-under-the-concentration-time curve up to 16 weeks (AUC0-16 weeks) of denosumab was 316 mcg·day/mL (SD = 101 mcg·day/mL).
No accumulation or change in denosumab pharmacokinetics with time was observed upon multiple dosing of 60 mg subcutaneously administered once every 6 months.
Denosumab pharmacokinetics were not affected by the formation of binding antibodies.
A population pharmacokinetic analysis was performed to evaluate the effects of demographic characteristics. This analysis showed no notable differences in pharmacokinetics with age (in postmenopausal women), race, or body weight (36 to 140 kg).
Seminal Fluid Pharmacokinetic Study
Serum and seminal fluid concentrations of denosumab were measured in 12 healthy male volunteers (age range: 43-65 years). After a single 60 mg subcutaneous administration of denosumab, the mean (± SD) Cmax values in the serum and seminal fluid samples were 6170 (± 2070) and 100 (± 81.9) ng/mL, respectively, resulting in a maximum seminal fluid concentration of approximately 2% of serum levels. The median (range) Tmax values in the serum and seminal fluid samples were 8.0 (7.9 to 21) and 21 (8.0 to 49) days, respectively. Among the subjects, the highest denosumab concentration in seminal fluid was 301 ng/mL at 22 days post-dose. On the first day of measurement (10 days post-dose), nine of eleven subjects had quantifiable concentrations in semen. On the last day of measurement (106 days post-dose), five subjects still had quantifiable concentrations of denosumab in seminal fluid, with a mean (± SD) seminal fluid concentration of 21.1 (± 36.5) ng/mL across all subjects (n = 12).
Drug Interactions
In a study of 19 postmenopausal women with low BMD and rheumatoid arthritis treated with etanercept (50 mg subcutaneous injection once weekly), a single-dose of denosumab (60 mg subcutaneous injection) was administered 7 days after the previous dose of etanercept. No clinically significant changes in the pharmacokinetics of etanercept were observed.
Cytochrome P450 substrates
In a study of 17 postmenopausal women with osteoporosis, midazolam (2 mg oral) was administered 2 weeks after a single-dose of denosumab (60 mg subcutaneous injection), which approximates the Tmax of denosumab. Denosumab did not affect the pharmacokinetics of midazolam, which is metabolized by cytochrome P450 3A4 (CYP3A4). This indicates that denosumab products should not alter the pharmacokinetics of drugs metabolized by CYP3A4 in postmenopausal women with osteoporosis.
Specific Populations
Gender: Mean serum denosumab concentration-time profiles observed in a study conducted in healthy men ≥ 50 years were similar to those observed in a study conducted in postmenopausal women using the same dose regimen.
Age: The pharmacokinetics of denosumab were not affected by age across all populations studied whose ages ranged from 28 to 87 years.
Race: The pharmacokinetics of denosumab were not affected by race.
Renal Impairment: In a study of 55 patients with varying degrees of renal function, including patients on dialysis, the degree of renal impairment had no effect on the pharmacokinetics of denosumab; thus, dose adjustment for renal impairment is not necessary.
Hepatic Impairment: No clinical studies have been conducted to evaluate the effect of hepatic impairment on the pharmacokinetics of denosumab products.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenicity
The carcinogenic potential of denosumab products has not been evaluated in long-term animal studies.
Mutagenicity
The genotoxic potential of denosumab products has not been evaluated.
Impairment of Fertility
Denosumab had no effect on female fertility or male reproductive organs in monkeys at doses that were 13- to 50-fold higher than the recommended human dose of 60 mg subcutaneously administered once every 6 months, based on body weight (mg/kg).
13.2. Animal Toxicology and/or Pharmacology
Denosumab products are an inhibitor of osteoclastic bone resorption via inhibition of RANKL.
In ovariectomized monkeys, once-monthly treatment with denosumab suppressed bone turnover and increased BMD and strength of cancellous and cortical bone at doses 50-fold higher than the recommended human dose of 60 mg administered once every 6 months, based on body weight (mg/kg). Bone tissue was normal with no evidence of mineralization defects, accumulation of osteoid, or woven bone.
Because the biological activity of denosumab in animals is specific to nonhuman primates, evaluation of genetically engineered (“knockout”) mice or use of other biological inhibitors of the RANK/RANKL pathway, namely OPG-Fc, provided additional information on the pharmacodynamic properties of denosumab products. RANK/RANKL knockout mice exhibited absence of lymph node formation, as well as an absence of lactation due to inhibition of mammary gland maturation (lobulo-alveolar gland development during pregnancy). Neonatal RANK/RANKL knockout mice exhibited reduced bone growth and lack of tooth eruption. A corroborative study in 2-week-old rats given the RANKL inhibitor OPG-Fc also showed reduced bone growth, altered growth plates, and impaired tooth eruption. These changes were partially reversible in this model when dosing with the RANKL inhibitors was discontinued.
14. Clinical Studies
14.1 Treatment of Postmenopausal Women with Osteoporosis
The efficacy and safety of denosumab in the treatment of postmenopausal osteoporosis was demonstrated in a 3-year, randomized, double-blind, placebo-controlled trial. Enrolled women had a baseline BMD T-score between -2.5 and -4.0 at either the lumbar spine or total hip. Women with other diseases (such as rheumatoid arthritis, osteogenesis imperfecta, and Paget’s disease) or on therapies that affect bone were excluded from this study. The 7808 enrolled women were aged 60 to 91 years with a mean age of 72 years. Overall, the mean baseline lumbar spine BMD T-score was -2.8, and 23% of women had a vertebral fracture at baseline. Women were randomized to receive subcutaneous injections of either placebo (N = 3906) or denosumab 60 mg (N = 3902) once every 6 months. All women received at least 1000 mg calcium and 400 IU vitamin D supplementation daily.
The primary efficacy variable was the incidence of new morphometric (radiologically-diagnosed) vertebral fractures at 3 years. Vertebral fractures were diagnosed based on lateral spine radiographs (T4-L4) using a semiquantitative scoring method. Secondary efficacy variables included the incidence of hip fracture and nonvertebral fracture, assessed at 3 years.
Effect on Vertebral Fractures
Denosumab significantly reduced the incidence of new morphometric vertebral fractures at 1, 2, and 3 years (p < 0.0001), as shown in Table 3. The incidence of new vertebral fractures at year 3 was 7.2% in the placebotreated women compared to 2.3% for the denosumab-treated women. The absolute risk reduction was 4.8% and relative risk reduction was 68% for new morphometric vertebral fractures at year 3.
Table 3. The Effect of Denosumab on the Incidence of New Vertebral Fractures in Postmenopausal Women:
| Proportion of Women with Fracture (%)+ | Absolute Risk Reduction ()* (95 CI) | Relative Risk Reduction ()* (95 CI) | ||
|---|---|---|---|---|
| Placebo N = 3691 (%) | Denosumab N = 3702 (%) | |||
| 0-1 Year | 2.2 | 0.9 | 1.4 (0.8, 1.9) | 61 (42, 74) |
| 0-2 Years | 5.0 | 1.4 | 3.5 (2.7, 4.3) | 71 (61, 79) |
| 0-3 Years | 7.2 | 2.3 | 4.8 (3.9, 5.8) | 68 (59, 74) |
+ Event rates based on crude rates in each interval.
* Absolute risk reduction and relative risk reduction based on Mantel-Haenszel method adjusting for age group variable.
Denosumab was effective in reducing the risk for new morphometric vertebral fractures regardless of age, baseline rate of bone turnover, baseline BMD, baseline history of fracture, or prior use of a drug for osteoporosis.
Effect on Hip Fractures
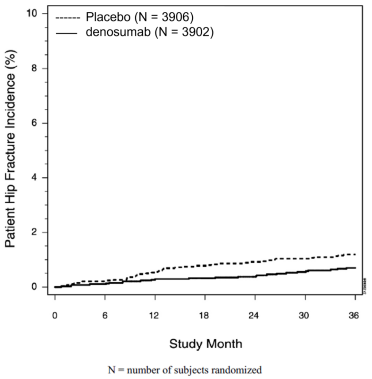
The incidence of hip fracture was 1.2% for placebo-treated women compared to 0.7% for denosumab-treated women at year 3. The age-adjusted absolute risk reduction of hip fractures was 0.3% with a relative risk reduction of 40% at 3 years (p = 0.04) (Figure 1).
Figure 1. Cumulative Incidence of Hip Fractures Over 3 Years:
Effect on Nonvertebral Fractures
Treatment with denosumab resulted in a significant reduction in the incidence of nonvertebral fractures (Table 4).
Table 4. The Effect of Denosumab on the Incidence of Nonvertebral Fractures at Year 3:
| Proportion of Women with Fracture (%)+ | Absolute Risk Reduction () (95 CI) | Relative Risk Reduction () (95 CI) | ||
|---|---|---|---|---|
| Placebo N = 3906 (%) | Denosumab N = 3902 (%) | |||
| Nonvertebral fracture1 | 8.0 | 6.5 | 1.5 (0.3, 2.7) | 20 (5, 33)* |
+ Event rates based on Kaplan-Meier estimates at 3 years.
1 Excluding those of the vertebrae (cervical, thoracic, and lumbar), skull, facial, mandible, metacarpus, and finger and toe phalanges.
* p-value = 0.01.
Effect on Bone Mineral Density (BMD)
Treatment with denosumab significantly increased BMD at all anatomic sites measured at 3 years. The treatment differences in BMD at 3 years were 8.8% at the lumbar spine, 6.4% at the total hip, and 5.2% at the femoral neck. Consistent effects on BMD were observed at the lumbar spine, regardless of baseline age, race, weight/body mass index (BMI), baseline BMD, and level of bone turnover.
After denosumab discontinuation, BMD returned to approximately baseline levels within 12 months.
Bone Histology and Histomorphometry
A total of 115 transiliac crest bone biopsy specimens were obtained from 92 postmenopausal women with osteoporosis at either month 24 and/or month 36 (53 specimens in denosumab group, 62 specimens in placebo group). Of the biopsies obtained, 115 (100%) were adequate for qualitative histology and 7 (6%) were adequate for full quantitative histomorphometry assessment.
Qualitative histology assessments showed normal architecture and quality with no evidence of mineralization defects, woven bone, or marrow fibrosis in patients treated with denosumab.
The presence of double tetracycline labeling in a biopsy specimen provides an indication of active bone remodeling, while the absence of tetracycline label suggests suppressed bone formation. In patients treated with denosumab, 35% had no tetracycline label present at the month 24 biopsy and 38% had no tetracycline label present at the month 36 biopsy, while 100% of placebo-treated patients had double label present at both time points. When compared to placebo, treatment with denosumab resulted in virtually absent activation frequency and markedly reduced bone formation rates. However, the long-term consequences of this degree of suppression of bone remodeling are unknown.
14.2 Treatment to Increase Bone Mass in Men with Osteoporosis
The efficacy and safety of denosumab in the treatment to increase bone mass in men with osteoporosis was demonstrated in a 1-year, randomized, double-blind, placebo-controlled trial. Enrolled men had a baseline BMD T-score between -2.0 and -3.5 at the lumbar spine or femoral neck. Men with a BMD T-score between -1.0 and -3.5 at the lumbar spine or femoral neck were also enrolled if there was a history of prior fragility fracture. Men with other diseases (such as rheumatoid arthritis, osteogenesis imperfecta, and Paget’s disease) or on therapies that may affect bone were excluded from this study. The 242 men enrolled in the study ranged in age from 31 to 84 years with a mean age of 65 years. Men were randomized to receive SC injections of either placebo (n = 121) or denosumab 60 mg (n = 121) once every 6 months. All men received at least 1000 mg calcium and at least 800 IU vitamin D supplementation daily.
Effect on Bone Mineral Density (BMD)
The primary efficacy variable was percent change in lumbar spine BMD from baseline to 1-year. Secondary efficacy variables included percent change in total hip, and femoral neck BMD from baseline to 1-year.
Treatment with denosumab significantly increased BMD at 1-year. The treatment differences in BMD at 1-year were 4.8% (+0.9% placebo, +5.7% denosumab; (95% CI: 4.0, 5.6); p < 0.0001) at the lumbar spine, 2.0% (+0.3% placebo, +2.4% denosumab) at the total hip, and 2.2% (0.0% placebo, +2.1% denosumab) at femoral neck. Consistent effects on BMD were observed at the lumbar spine regardless of baseline age, race, BMD, testosterone concentrations, and level of bone turnover.
Bone Histology and Histomorphometry
A total of 29 transiliac crest bone biopsy specimens were obtained from men with osteoporosis at 12 months (17 specimens in denosumab group, 12 specimens in placebo group). Of the biopsies obtained, 29 (100%) were adequate for qualitative histology and, in denosumab patients, 6 (35%) were adequate for full quantitative histomorphometry assessment. Qualitative histology assessments showed normal architecture and quality with no evidence of mineralization defects, woven bone, or marrow fibrosis in patients treated with denosumab. The presence of double tetracycline labeling in a biopsy specimen provides an indication of active bone remodeling, while the absence of tetracycline label suggests suppressed bone formation. In patients treated with denosumab, 6% had no tetracycline label present at the month 12 biopsy, while 100% of placebo-treated patients had double label present. When compared to placebo, treatment with denosumab resulted in markedly reduced bone formation rates. However, the long-term consequences of this degree of suppression of bone remodeling are unknown.
14.3 Treatment of Glucocorticoid-Induced Osteoporosis
The efficacy and safety of denosumab in the treatment of patients with glucocorticoid-induced osteoporosis was assessed in the 12-month primary analysis of a 2-year, randomized, multicenter, double-blind, parallel-group, active-controlled study (NCT 01575873) of 795 patients (70% women and 30% men) aged 20 to 94 years (mean age of 63 years) treated with greater than or equal to 7.5 mg/day oral prednisone (or equivalent) for < 3 months prior to study enrollment and planning to continue treatment for a total of at least 6 months (glucocorticoidinitiating subpopulation; n = 290) or ≥ 3 months prior to study enrollment and planning to continue treatment for a total of at least 6 months (glucocorticoid-continuing subpopulation, n = 505). Enrolled patients < 50 years of age were required to have a history of osteoporotic fracture. Enrolled patients ≥ 50 years of age who were in the glucocorticoid-continuing subpopulation were required to have a baseline BMD T-score of ≤ -2.0 at the lumbar spine, total hip, or femoral neck; or a BMD T-score ≤ -1.0 at the lumbar spine, total hip, or femoral neck and a history of osteoporotic fracture.
Patients were randomized (1:1) to receive either an oral daily bisphosphonate (active-control, risedronate 5 mg once daily) (n = 397) or denosumab 60 mg subcutaneously once every 6 months (n = 398) for one year. Randomization was stratified by gender within each subpopulation. Patients received at least 1000 mg calcium and 800 IU vitamin D supplementation daily.
Effect on Bone Mineral Density (BMD)
In the glucocorticoid-initiating subpopulation, denosumab significantly increased lumbar spine BMD compared to the active-control at one year (Active-control 0.8%, denosumab 3.8%) with a treatment difference of 2.9% (p < 0.001). In the glucocorticoid-continuing subpopulation, denosumab significantly increased lumbar spine BMD compared to active-control at one year (Active-control 2.3%, denosumab 4.4%) with a treatment difference of 2.2% (p < 0.001). Consistent effects on lumbar spine BMD were observed regardless of gender; race; geographic region; menopausal status; and baseline age, lumbar spine BMD T-score, and glucocorticoid dose within each subpopulation.
Bone Histology
Bone biopsy specimens were obtained from 17 patients (11 in the active-control treatment group and 6 in the denosumab treatment group) at Month 12. Of the biopsies obtained, 17 (100%) were adequate for qualitative histology. Qualitative assessments showed bone of normal architecture and quality without mineralization defects or bone marrow abnormality. The presence of double tetracycline labeling in a biopsy specimen provides an indication of active bone remodeling, while the absence of tetracycline label suggests suppressed bone formation. In patients treated with active-control, 100% of biopsies had tetracycline label. In patients treated with denosumab, 1 (33%) had tetracycline label and 2 (67%) had no tetracycline label present at the 12-month biopsy. Evaluation of full quantitative histomorphometry including bone remodeling rates was not possible in the glucocorticoid-induced osteoporosis population treated with denosumab. The long-term consequences of this degree of suppression of bone remodeling in glucocorticoid-treated patients is unknown.
14.4 Treatment of Bone Loss in Men with Prostate Cancer
The efficacy and safety of denosumab in the treatment of bone loss in men with nonmetastatic prostate cancer receiving androgen deprivation therapy (ADT) were demonstrated in a 3-year, randomized (1:1), double-blind, placebo-controlled, multinational study. Men less than 70 years of age had either a BMD T-score at the lumbar spine, total hip, or femoral neck between -1.0 and -4.0, or a history of an osteoporotic fracture. The mean baseline lumbar spine BMD T-score was -0.4, and 22% of men had a vertebral fracture at baseline. The 1468 men enrolled ranged in age from 48 to 97 years (median 76 years). Men were randomized to receive subcutaneous injections of either placebo (n = 734) or denosumab 60 mg (n = 734) once every 6 months for a total of 6 doses. Randomization was stratified by age (< 70 years vs. ≥ 70 years) and duration of ADT at trial entry (≤ 6 months vs. > 6 months). Seventy-nine percent of patients received ADT for more than 6 months at study entry. All men received at least 1000 mg calcium and 400 IU vitamin D supplementation daily.
Effect on Bone Mineral Density (BMD)
The primary efficacy variable was percent change in lumbar spine BMD from baseline to month 24. An additional key secondary efficacy variable was the incidence of new vertebral fracture through month 36 diagnosed based on x-ray evaluation by two independent radiologists. Lumbar spine BMD was higher at 2 years in denosumab-treated patients as compared to placebo-treated patients [-1.0% placebo, +5.6% denosumab; treatment difference 6.7% (95% CI: 6.2, 7.1); p < 0.0001].
With approximately 62% of patients followed for 3 years, treatment differences in BMD at 3 years were 7.9% (-1.2% placebo, +6.8% denosumab) at the lumbar spine, 5.7% (-2.6% placebo, +3.2% denosumab) at the total hip, and 4.9% (-1.8% placebo, +3.0% denosumab) at the femoral neck. Consistent effects on BMD were observed at the lumbar spine in relevant subgroups defined by baseline age, BMD, and baseline history of vertebral fracture.
Effect on Vertebral Fractures
Denosumab significantly reduced the incidence of new vertebral fractures at 3 years (p = 0.0125), as shown in Table 5.
Table 5. The Effect of Denosumab on the Incidence of New Vertebral Fractures in Men with Nonmetastatic Prostate Cancer:
| Proportion of Men with Fracture (%)+ | Absolute Risk Reduction ()* (95 CI) | Relative Risk Reduction ()* (95 CI) | ||
|---|---|---|---|---|
| Placebo N = 673 (%) | Denosumab N = 679 (%) | |||
| 0-1 Year | 1.9 | 0.3 | 1.6 (0.5, 2.8) | 85 (33, 97) |
| 0-2 Years | 3.3 | 1.0 | 2.2 (0.7, 3.8) | 69 (27, 86) |
| 0-3 Years | 3.9 | 1.5 | 2.4 (0.7, 4.1) | 62 (22, 81) |
+ Event rates based on crude rates in each interval.
* Absolute risk reduction and relative risk reduction based on Mantel-Haenszel method adjusting for age group and ADT duration variables.
14.5 Treatment of Bone Loss in Women with Breast Cancer
The efficacy and safety of denosumab in the treatment of bone loss in women receiving adjuvant aromatase inhibitor (AI) therapy for breast cancer was assessed in a 2-year, randomized (1:1), double-blind, placebo-controlled, multinational study. Women had baseline BMD T-scores between -1.0 to -2.5 at the lumbar spine, total hip, or femoral neck, and had not experienced fracture after age 25. The mean baseline lumbar spine BMD T-score was -1.1, and 2.0% of women had a vertebral fracture at baseline. The 252 women enrolled ranged in age from 35 to 84 years (median 59 years). Women were randomized to receive subcutaneous injections of either placebo (n = 125) or denosumab 60 mg (n = 127) once every 6 months for a total of 4 doses. Randomization was stratified by duration of adjuvant AI therapy at trial entry (≤ 6 months vs. > 6 months). Sixty-two percent of patients received adjuvant AI therapy for more than 6 months at study entry. All women received at least 1000 mg calcium and 400 IU vitamin D supplementation daily.
Effect on Bone Mineral Density (BMD)
The primary efficacy variable was percent change in lumbar spine BMD from baseline to month 12. Lumbar spine BMD was higher at 12 months in denosumab-treated patients as compared to placebo-treated patients [-0.7% placebo, +4.8% denosumab; treatment difference 5.5% (95% CI: 4.8, 6.3); p < 0.0001].
With approximately 81% of patients followed for 2 years, treatment differences in BMD at 2 years were 7.6% (-1.4% placebo, +6.2% denosumab) at the lumbar spine, 4.7% (-1.0% placebo, +3.8% denosumab) at the total hip, and 3.6% (-0.8% placebo, +2.8% denosumab) at the femoral neck.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.