JULUCA Film-coated tablet Ref.[51355] Active ingredients: Dolutegravir Dolutegravir and Rilpivirine Rilpivirine
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: ViiV Healthcare BV, Van Asch van Wijckstraat 55H, 3811 LP Amersfoort, Netherlands
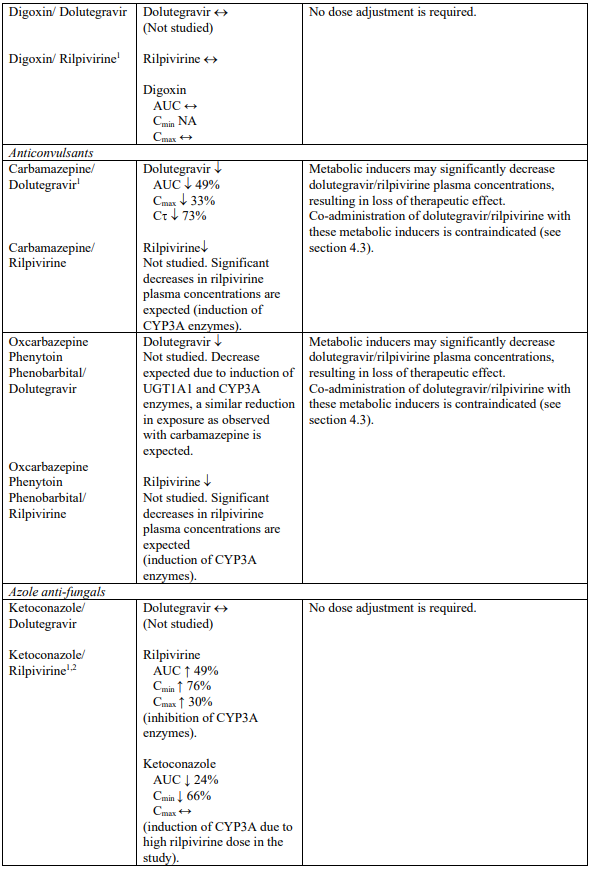
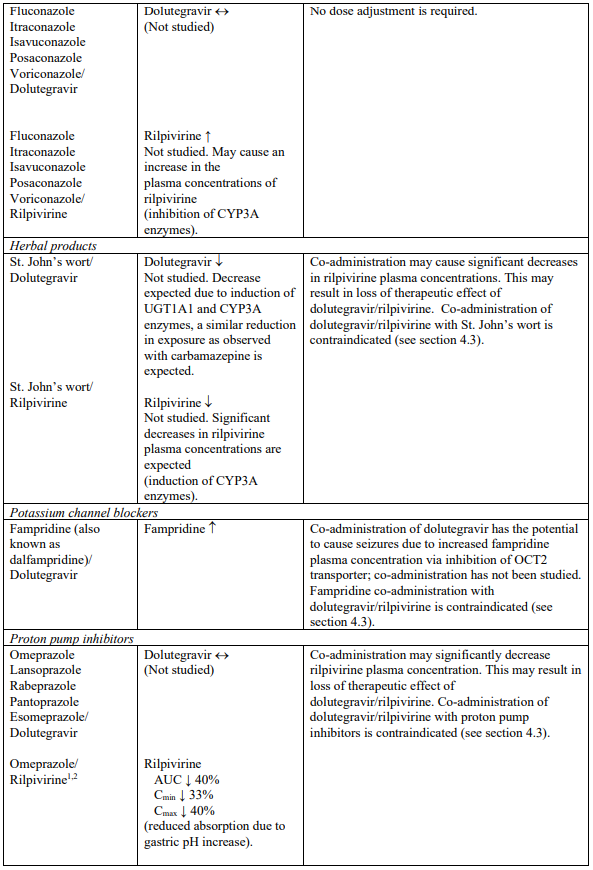
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
Co-administration with the following medicinal products:
- fampridine (also known as dalfampridine);
- carbamazepine, oxcarbazepine, phenobarbital, phenytoin;
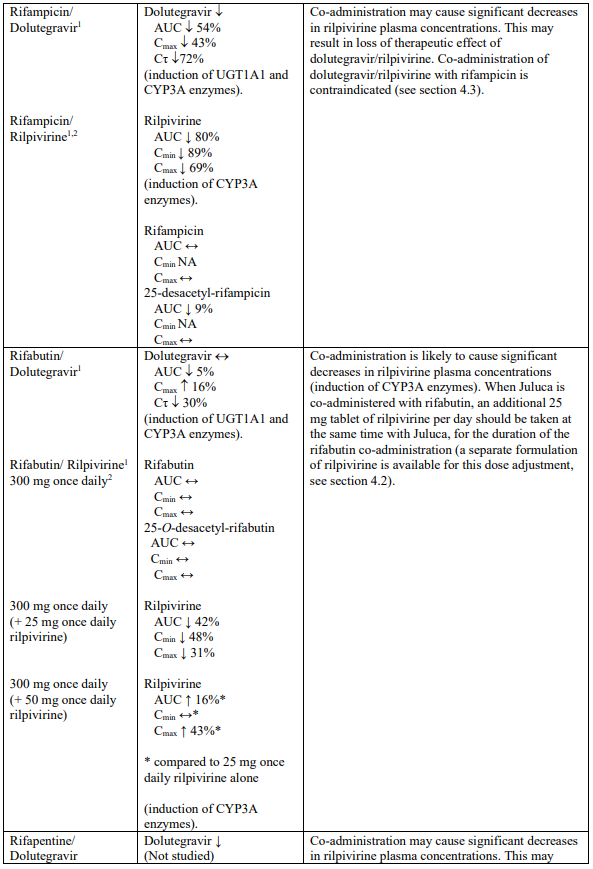
- rifampicin, rifapentine;
- proton pump inhibitors, such as omeprazole, esomeprazole, lansoprazole, pantoprazole, rabeprazole;
- systemic dexamethasone, except as a single dose treatment;
- St John’s wort (Hypericum perforatum).
4.4. Special warnings and precautions for use
Hypersensitivity reactions
Hypersensitivity reactions have been reported with dolutegravir, and were characterised by rash, constitutional findings, and sometimes, organ dysfunction, including severe liver reactions. dolutegravir/rilpivirine should be discontinued immediately if signs or symptoms of hypersensitivity reactions develop (including, but not limited to, severe rash or rash accompanied by raised liver enzymes, fever, general malaise, fatigue, muscle or joint aches, blisters, oral lesions, conjunctivitis, facial oedema, eosinophilia, angioedema). Clinical status including liver aminotransferases and bilirubin should be monitored. Delay in stopping treatment with dolutegravir/rilpivirine after the onset of hypersensitivity may result in a life-threatening allergic reaction.
Weight and metabolic parameters
An increase in weight and in levels of blood lipids and glucose may occur during antiretroviral therapy. Such changes may in part be linked to disease control and lifestyle. For lipids and weight, there is in some cases evidence for a treatment effect. For monitoring of blood lipids and glucose reference is made to established HIV treatment guidelines. Lipid disorders should be managed as clinically appropriate.
Cardiovascular
At supra-therapeutic doses (75 and 300 mg once daily), rilpivirine has been associated with prolongation of the QTc interval of the electrocardiogram (ECG) (see sections 4.5 and 5.1). Rilpivirine at the recommended dose of 25 mg once daily is not associated with a clinically relevant effect on QTc. Dolutegravir/rilpivirine should be used with caution when co-administered with medicinal products with a known risk of Torsade de Pointes.
Opportunistic infections
Patients should be advised that dolutegravir/rilpivirine does not cure HIV infection and that they may still develop opportunistic infections and other complications of HIV infection. Therefore, patients should remain under close clinical observation by physicians experienced in the treatment of these associated HIV diseases.
Osteonecrosis
Although the aetiology is considered to be multifactorial (including corticosteroid use, biphosphonates, alcohol consumption, severe immunosuppression, higher body mass index), cases of osteonecrosis have been reported in patients with advanced HIV-disease and/or long-term exposure to CART. Patients should be advised to seek medical advice if they experience joint aches and pain, joint stiffness or difficulty in movement.
Patients with hepatitis B or C
No clinical data are available in patients with hepatitis B co-infection. Physicians should refer to current treatment guidelines for the management of HIV infection in patients co-infected with hepatitis B virus. Limited data is available in patients with hepatitis C co-infection. A higher incidence of liver chemistry elevations (Grade 1) were observed in patients treated with dolutegravir and rilpivirine co-infected with hepatitis C compared to those who were not co-infected. Monitoring of liver function is recommended in patients with hepatitis B and/or C co-infection.
Interactions with other medicinal products
Dolutegravir/rilpivirine should not be administered with other antiretroviral medicinal products for the treatment of HIV (see section 4.5).
Juluca should not be taken with any other medicinal product containing dolutegravir or rilpivirine, except in case of co-administration with rifabutin (see section 4.5).
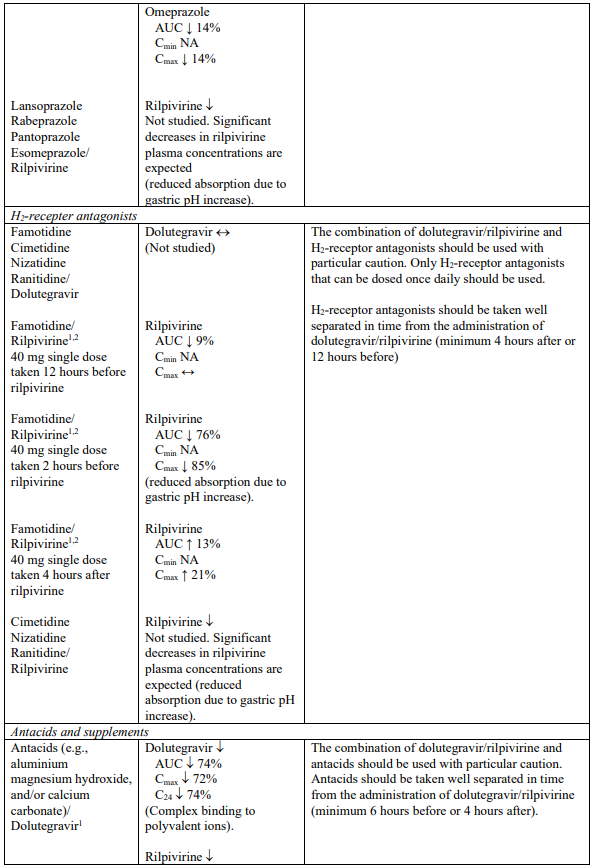
H2-receptor antagonists
Dolutegravir/rilpivirine should not be co-administered at the same time as H2-receptor antagonists. These medicinal products are recommended to be administered 12 hours before or 4 hours after dolutegravir/rilpivirine (see section 4.5).
Antacids
Dolutegravir/rilpivirine should not be co-administered at the same time as antacids. These medicinal products are recommended to be administered 6 hours before or 4 hours after dolutegravir/rilpivirine (see section 4.5).
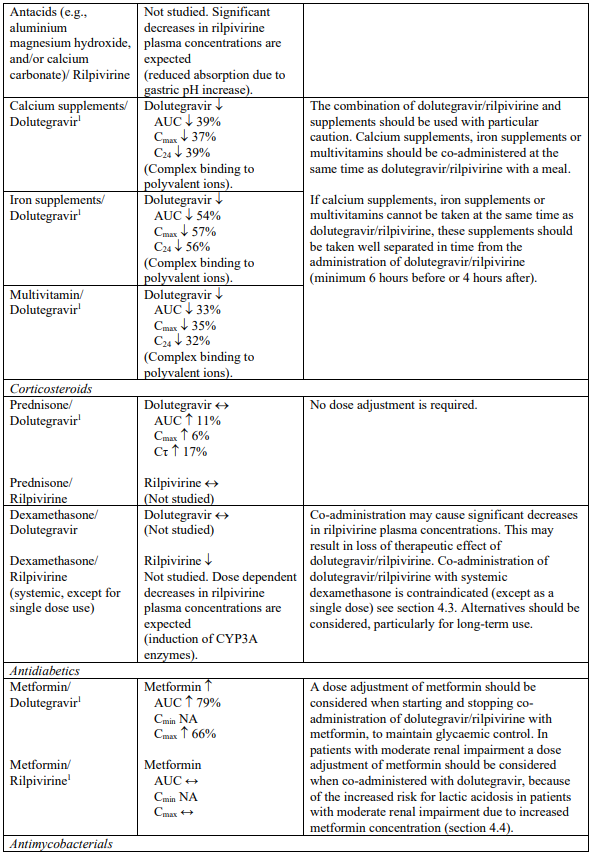
Supplements and multivitamins
Calcium or iron supplements, or multivitamins should be co-administered at the same time as dolutegravir/rilpivirine, with a meal. If calcium or iron supplements, or multivitamins cannot be taken at the same time as dolutegravir/rilpivirine, these supplements are recommended to be administered 6 hours before or 4 hours after taking dolutegravir/rilpivirine (see section 4.5).
Metformin
Dolutegravir increased metformin concentrations. A dose adjustment of metformin should be considered when starting and stopping co-administration of dolutegravir/rilpivirine with metformin, to maintain glycaemic control (see section 4.5). Metformin is eliminated renally and therefore it is of importance to monitor renal function when co-treated with dolutegravir/rilpivirine. This combination may increase the risk for lactic acidosis in patients with moderate renal impairment (stage 3a creatinine clearance [CrCl] 45-59 mL/min) and a cautious approach is recommended. Reduction of the metformin dose should be highly considered.
Immune Reconstitution Syndrome
In HIV-infected patients with severe immune deficiency at the time of institution of combination antiretroviral therapy (CART), an inflammatory reaction to asymptomatic or residual opportunistic pathogens may arise and cause serious clinical conditions, or aggravation of symptoms. Typically, such reactions have been observed within the first few weeks or months of initiation of CART. Relevant examples are cytomegalovirus retinitis, generalised and/or focal mycobacterial infections, and Pneumocystis jirovecii pneumonia. Any inflammatory symptoms should be evaluated and treatment instituted when necessary. Autoimmune disorders (such as Graves' disease and autoimmune hepatitis) have also been reported to occur in the setting of immune reconstitution, however, the reported time to onset is more variable and these events can occur many months after initiation of treatment.
Excipients
Juluca contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
This medicine contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
4.5. Interaction with other medicinal products and other forms of interaction
Juluca is intended for use as a complete regimen for the treatment of HIV-1 infection and should not be administered with other antiretroviral medicinal products for the treatment of HIV. Therefore, information regarding drug-drug interactions with other antiretroviral medicinal products is not provided. Juluca contains dolutegravir and rilpivirine, therefore any interactions identified with these active substances are relevant to Juluca. Interaction studies have only been performed in adults.
Effect of other medicinal products on the pharmacokinetics of dolutegravir and rilpivirine
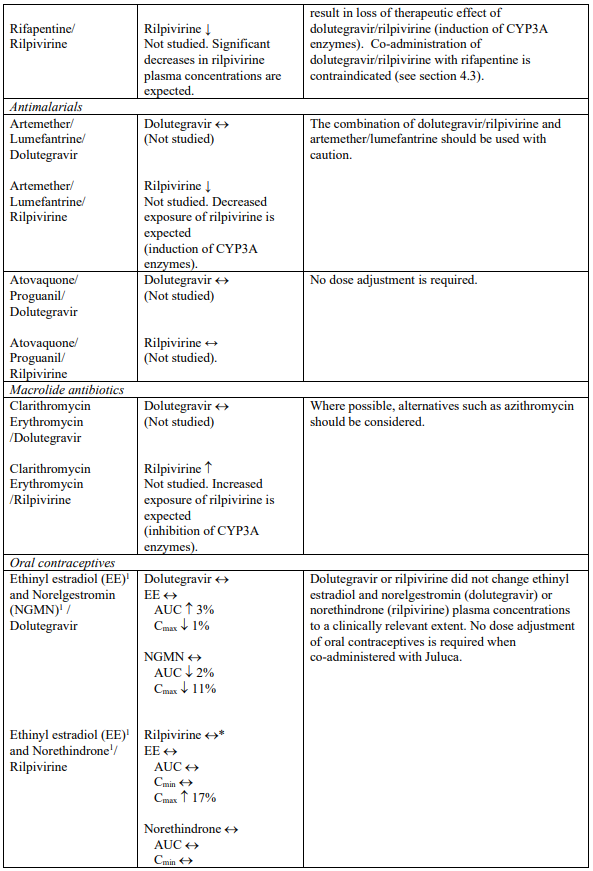
Dolutegravir is eliminated mainly through metabolism by uridine diphosphate glucuronosyl transferase (UGT)1A1. Dolutegravir is also a substrate of UGT1A3, UGT1A9, cytochrome P450 (CYP)3A4, Pglycoprotein (P-gp), and breast cancer resistance protein (BCRP); therefore medicinal products that induce those enzymes may decrease dolutegravir plasma concentration and reduce the therapeutic effect of dolutegravir (see Table 1). Co-administration of dolutegravir/rilpivirine and other medicinal products that inhibit these enzymes may increase dolutegravir plasma concentration (see Table 1).
The absorption of dolutegravir is reduced by certain anti-acid medicinal products (see Table 1).
Rilpivirine is primarily metabolised by CYP3A. Medicinal products that induce or inhibit CYP3A may thus affect the clearance of rilpivirine (see section 5.2). Co-administration of dolutegravir/rilpivirine with medicinal products that induce CYP3A may result in decreased plasma concentrations of rilpivirine, which could reduce the therapeutic effect of dolutegravir/rilpivirine (see Table 1). Co-administration of dolutegravir/rilpivirine with medicinal products that inhibit CYP3A may result in increased plasma concentrations of rilpivirine (see Table 1). In patients with severe renal impairment or end stage renal disease, the combination of dolutegravir/rilpivirine with a strong CYP3A inhibitor should only be used if the benefit outweighs the risk (see section 4.2).
Co-administration of dolutegravir/rilpivirine with medicinal products that increase gastric pH may result in decreased plasma concentrations of rilpivirine which could potentially reduce the therapeutic effect of dolutegravir/rilpivirine.
Effect of dolutegravir and rilpivirine on the pharmacokinetics of other medicinal products
Based on in vivo and/or in vitro data, dolutegravir is not expected to affect the pharmacokinetics of medicinal products that are substrates of any major enzyme or transporter such as CYP3A4, CYP2C9 and P-gp (for more information see section 5.2).
In vitro, dolutegravir inhibited the renal organic cation transporter 2 (OCT2) and multidrug and toxin extrusion transporter 1 (MATE1). In vivo, a 10-14% decrease of creatinine clearance (secretory fraction is dependent on OCT2 and MATE1 transport) was observed in patients. In vivo, dolutegravir may increase plasma concentrations of medicinal products in which excretion is dependent upon OCT2 and/or MATE1 (e.g. fampridine [also known as dalfampridine], metformin) (see Table 1 and sections 4.3 and 4.4).
In vitro, dolutegravir inhibited the renal uptake transporters, organic anion transporters (OAT)1 and OAT3. Based on the lack of effect on the in vivo pharmacokinetics of the OAT substrate tenofovir, in vivo inhibition of OAT1 is unlikely. Inhibition of OAT3 has not been studied in vivo. Dolutegravir may increase plasma concentrations of medicinal products in which excretion is dependent upon OAT3.
Rilpivirine 25 mg once daily is not likely to have a clinically relevant effect on the exposure of medicinal products metabolised by CYP enzymes.
Rilpivirine inhibits P-gp in vitro (IC50 is 9.2 μM). In a clinical study, rilpivirine did not significantly affect the pharmacokinetics of digoxin. However, it may not be completely excluded that rilpivirine can increase the exposure to other medicinal products transported by P-gp that are more sensitive to intestinal P-gp inhibition, e.g. dabigatran etexilate.
Rilpivirine is an in vitro inhibitor of the transporter MATE-2K with an IC50 of <2.7 nM. The clinical implications of this finding are currently unknown.
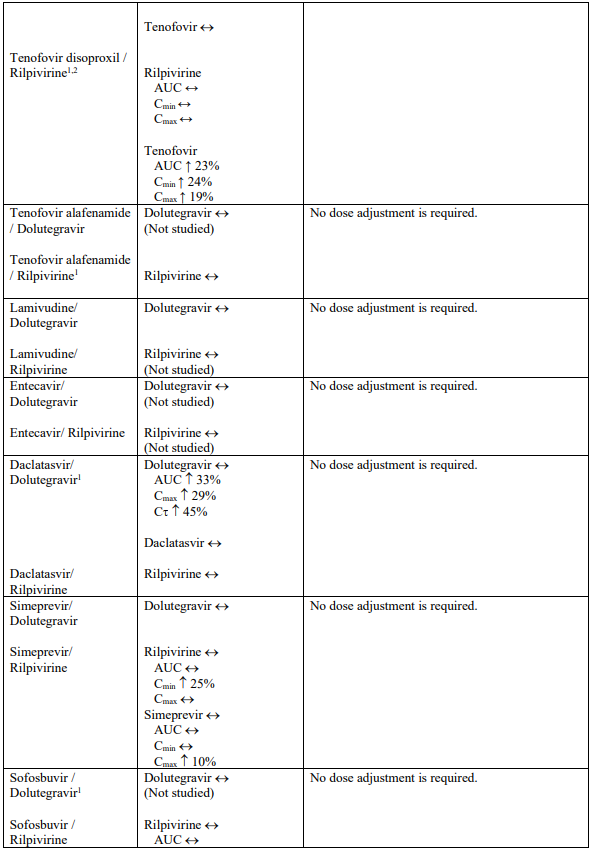
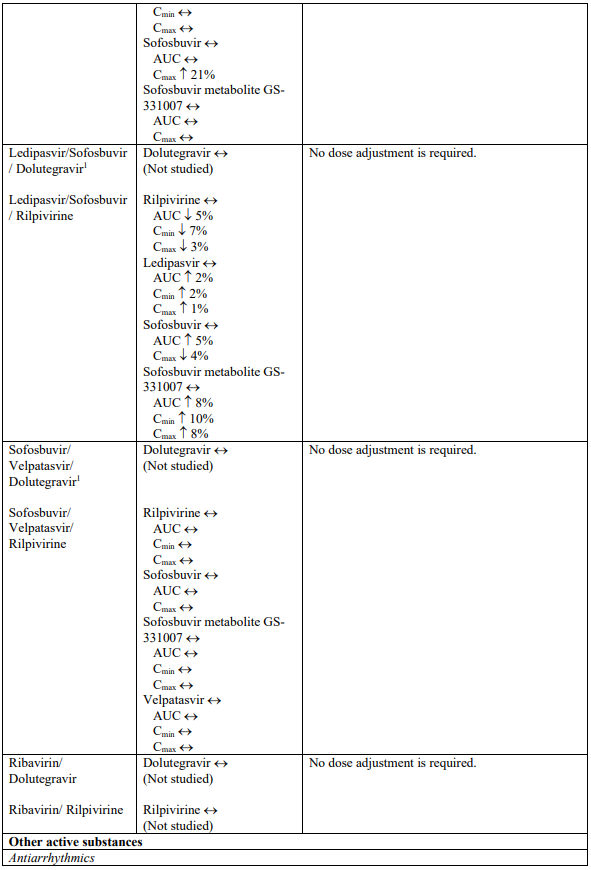
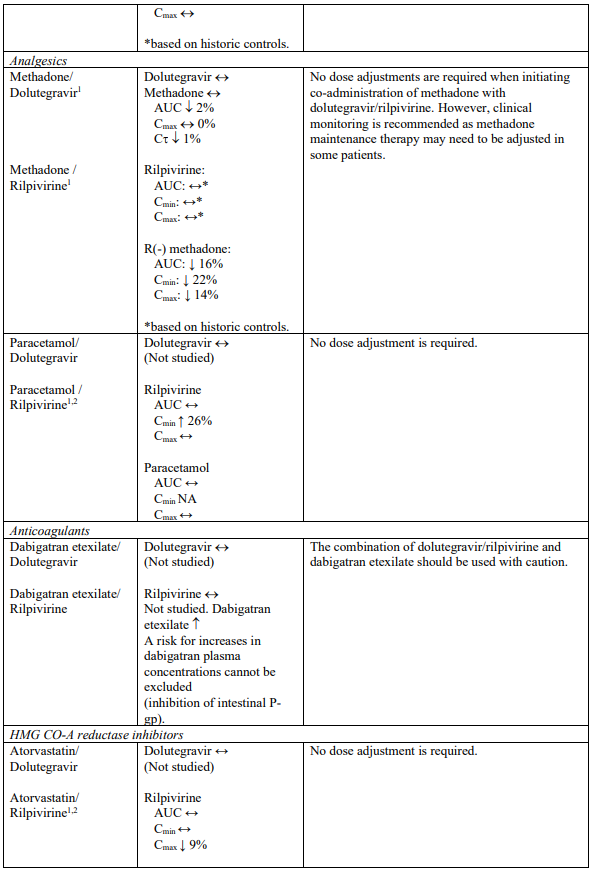
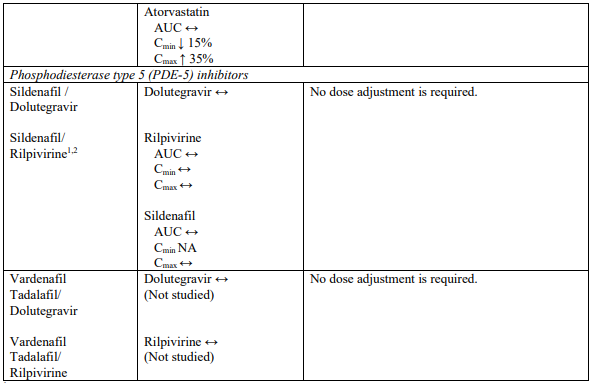
Interaction table
Selected established and theoretical interactions between dolutegravir, rilpivirine and co-administered medicinal products are listed in Table 1. (increase is indicated as “↑”, decrease as “↓”, no change as “↔”, area under the concentration versus time curve as “AUC”, maximum observed concentration as "Cmax", minimum observed concentration as "Cmin"concentration at end of dosing interval as “Cτ”).
Table 1. Drug Interactions:
1 The interaction between dolutegravir and/or rilpivirine and the medicinal product was evaluated in a clinical study. All other drug-drug interactions shown are predicted.
2 This interaction study has been performed with a dose higher than the recommended dose for rilpivirine assessing the maximal effect on the co-administered medicinal product.
NA = Not applicable
h3/ QT prolonging medicinal products
There is limited information available on the potential for a pharmacodynamic interaction between rilpivirine and medicinal products that prolong the QTc interval of the ECG. In a study of healthy subjects, supratherapeutic doses of rilpivirine (75 mg once daily and 300 mg once daily) have been shown to prolong the QTc interval of the ECG (see section 5.1). Dolutegravir/rilpivirine should be used with caution when coadministered with a medicinal product with a known risk of Torsade de Pointes.
4.6. Fertility, pregnancy and lactation
Women of childbearing potential
Women of childbearing potential should be counselled about the potential risk of neural tube defects with dolutegravir (a component of Juluca, see below), including consideration of effective contraceptive measures.
If a woman plans pregnancy, the benefits and the risks of continuing treatment with Juluca should be discussed with the patient.
Pregnancy
Lower exposures of dolutegravir and rilpivirine were observed during pregnancy (see sections 5.1, 5.2). In phase 3 studies, lower rilpivirine exposure, similar to that seen during pregnancy, has been associated with an increased risk of virological failure. The use of Juluca during pregnancy is not recommended.
The safety and efficacy of a dual regimen has not been studied in pregnancy.
Human experience from a birth outcome surveillance study in Botswana shows a small increase of neural tube defects; 7 cases in 3,591 deliveries (0.19%; 95% CI 0.09%, 0.40%) to mothers taking dolutegravircontaining regimens at the time of conception compared to 21 cases in 19,361 deliveries (0.11%: 95% CI 0.07%, 0.17%) to women exposed to non-dolutegravir regimens at the time of conception.
The incidence of neural tube defects in the general population ranges from 0.5-1 case per 1,000 live births (0.05-0.1%). Most neural tube defects occur within the first 4 weeks of embryonic development after conception (approximately 6 weeks after the last menstrual period).
Data analysed from the Antiretroviral Pregnancy Registry do not indicate an increased risk of major birth defects in over 600 women exposed to dolutegravir during pregnancy but are currently insufficient to address the risk of neural tube defects.
In animal reproductive toxicology studies with dolutegravir, no adverse development outcomes, including neural tube defects, were identified (see section 5.3).
More than 1000 outcomes from exposure to dolutegravir during second and third trimester pregnancy indicate no evidence of increased risk of foetal/neonatal toxicity.
Dolutegravir crosses the placenta in humans. In pregnant women living with HIV, the median foetal umbilical cord concentration of dolutegravir was approximately 1.3-fold greater compared with the maternal peripheral plasma concentration.
There is insufficient information on the effects of dolutegravir on neonates.
Animal studies with rilpivirine do not indicate direct or indirect harmful effects with respect to reproductive toxicity (see section 5.3).
Breast-feeding
It is unknown if rilpivirine is excreted in human milk. Available toxicological data in animals has shown excretion of rilpivirine in milk. Dolutegravir is excreted in human milk in small amounts (a median dolutegravir breast milk to maternal plasma ratio of 0.033 has been shown). There is insufficent information on the effects of dolutegravir in newborns/infants.
It is recommended that women living with HIV do not breast-feed their infants in order to avoid transmission of HIV.
Fertility
There are no data on the effects of dolutegravir or rilpivirine on human male or female fertility. Animal studies indicate no clinically relevant effects on male or female fertility (see section 5.3).
4.7. Effects on ability to drive and use machines
Juluca has no or negligible influence on the ability to drive and use machines. Patients should be informed that fatigue, dizziness and somnolence have been reported during treatment with the components of Juluca. The clinical status of the patient and the adverse reaction profile of Juluca should be borne in mind when considering the patient’s ability to drive or operate machinery.
4.8. Undesirable effects
Summary of the safety profile
The most frequently reported adverse reactions with Juluca (from clinical studies – see section 5.1) were diarrhoea (2%) and headache (2%).
The most severe adverse reaction, related to the treatment with dolutegravir (from pooled Phase IIb and Phase III clinical studies), seen in an individual patient, was a hypersensitivity reaction that included rash and severe liver effects (see section 4.4).
Tabulated list of adverse reactions
The sources of information for the safety database include 2 identical, randomised, open-label studies SWORD-1 and SWORD-2 (see section 5.1), pooled studies from individual components and post-marketing experience.
The adverse reactions considered at least possibly related to treatment with the components of Juluca from clinical studies and post-marketing experience are listed in Table 2 by body system, organ class and frequency. Frequencies are defined as very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1 000 to <1/100), rare (≥1/10 000 to <1/1 000), very rare (<1/10 000), not known (cannot be estimated from the available data).
Table 2. Tabulated list of adverse reactions to Juluca based on clinical study and postmarketing experience with Juluca and its individual components:
| System organ class (SOC) | Frequency category* | Adverse drug reactions |
|---|---|---|
| Blood and lymphatic systems disorders | common | decreased white blood cell count decreased haemoglobin decreased platelet count |
| Immune system disorders | uncommon | hypersensitivity (see section 4.4) |
| not known | immune reconstitution syndrome | |
| Metabolism and nutrition disorders | very common | increased total cholesterol (fasted) increased LDL cholesterol (fasted) |
| common | decreased appetite increased triglycerides (fasted) | |
| Psychiatric disorders | very common | insomnia |
| common | abnormal dreams depression sleep disorders depressed mood anxiety | |
| uncommon | suicidal ideation or suicide attempt (particularly in patients with a pre-existing history of depression or psychiatric illness), panic attack | |
| rare | completed suicide (particularly in patients with a preexisting history of depression or psychiatric illness) | |
| Nervous system disorders | very common | headache dizziness |
| common | somnolence | |
| Gastrointestinal disorders | very common | nausea increased pancreatic amylase diarrhoea |
| common | abdominal pain vomiting flatulence increased lipase abdominal discomfort upper abdominal pain dry mouth | |
| Hepatobiliary disorders | very common | increased transaminases (alanine aminotransferase (ALT) and/or aspartate aminotransferase (AST) elevations) |
| common | increased bilirubin | |
| uncommon | hepatitis | |
| rare | acute hepatic failure** | |
| Skin and subcutaneous tissue disorders | common | rash pruritus |
| Musculoskeletal and connective tissue disorders | uncommon | arthralgia myalgia |
| General disorders and administration site conditions | common | fatigue |
| Investigations | common | creatine phosphokinase (CPK) elevations, weight increased |
* Frequencies are assigned based on the maximum frequencies observed in the pooled SWORD studies or studies with the individual components
** This adverse reaction was identified through post-marketing surveillance for dolutegravir in combination with other ARVs. The frequency category of rare was estimated based on postmarketing reports.
Description of selected adverse reactions
Changes in laboratory biochemistries
Dolutegravir or rilpivirine have been associated with increases in serum creatinine occurring in the first week of treatment when administered with other antiretroviral medicinal products. Increases in serum creatinine occurred within the first four weeks of treatment with dolutegravir/rilpivirine and remained stable through 148 weeks. A mean change from baseline of 9.86 µmol/L (SD 10.4 µmol/L) was observed after 148 weeks treatment. These changes are related to inhibition of active transport, and are not considered to be clinically relevant as they do not reflect a change in glomerular filtration rate.
Metabolic parameters
Weight and levels of blood lipids and glucose may increase during antiretroviral therapy (see section 4.4).
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.