KADIAN Capsule extended-release Ref.[10855] Active ingredients: Morphine
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
KADIAN (morphine sulfate) extended-release capsules, an opioid agonist, are for oral use and contain pellets of morphine sulfate.
Each KADIAN extended-release capsule contains either 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, 80 mg, 100 mg, or 200 mg of morphine sulfate, USP and the following inactive ingredients common to all strengths: hypromellose, ethylcellulose, methacrylic acid copolymer, polyethylene glycol, diethyl phthalate, talc, corn starch, and sucrose.
The capsule shells contain gelatin, silicon dioxide, sodium lauryl sulfate, titanium dioxide, and black ink, D&C red #28, FD&C blue #1 (10 mg), D&C yellow #10 (20 mg), FD&C red #3, FD&C blue #1 (30 mg), D&C yellow #10, FD&C blue #1, FD&C red #3 (40 mg), D&C red #28, FD&C red #40, FD&C blue #1 (50 mg), D&C red #28, FD&C red #40, FD&C blue #1 (60 mg), FD&C blue #1, FD&C red #40, FD&C yellow #6 (80 mg), D&C yellow #10, FD&C blue #1 (100 mg) black iron oxide, yellow iron oxide, red iron oxide (200 mg). The imprint ink contains black iron oxide, potassium hydroxide, propylene glycol, and shellac.
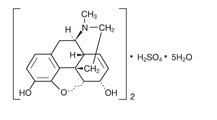
The chemical name of morphine sulfate is 7,8-didehydro-4,5 α-epoxy-17-methyl-morphinan-3,6 α-diol sulfate (2:1) (salt) pentahydrate. The empirical formula is (C17H19NO3)2•H2SO4•5H2O and its molecular weight is 758.85.
Morphine sulfate is an odorless, white, crystalline powder with a bitter taste. It has a solubility of 1 in 21 parts of water and 1 in 1000 parts of alcohol, but is practically insoluble in chloroform or ether. The octanol: water partition coefficient of morphine is 1.42 at physiologic pH and the pKb is 7.9 for the tertiary nitrogen (mostly ionized at pH 7.4).
Its structural formula is:
| Dosage Forms and Strengths |
|---|
|
Extended-release capsules: 10 mg, 20 mg, 30 mg, 40 mg, 50 mg, 60 mg, 80 mg, 100 mg, 200 mg. KADIAN contains white to off-white or tan colored polymer coated pellets, have an outer opaque capsule with colors as identified below and are available in nine dose strengths: Each 10 mg extended-release capsule has a light blue opaque cap printed with “KADIAN” and a light blue opaque body printed with “10 mg”. |
| How Supplied | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
KADIAN extended-release capsules contain white to off-white or tan colored polymer coated extended-release pellets of morphine sulfate and are available in nine dose strengths.
Distributed by: Allergan USA, Inc., Madison, NJ 07940 |
Drugs
| Drug | Countries | |
|---|---|---|
| KADIAN | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.