KCENTRA Powder for solution for infusion, lyophilized Ref.[50986] Active ingredients: Coagulation factor IX complex Protein C Protein S
Source: FDA, National Drug Code (US) Revision Year: 2023
12.1. Mechanism of Action
KCENTRA contains the Vitamin K-dependent coagulation Factors II (FII), VII (FVII), IX (FIX), and X (FX), together known as the Prothrombin Complex, and the antithrombotic Protein C and Protein S.
A dose-dependent acquired deficiency of the Vitamin K-dependent coagulation factors occurs during Vitamin K antagonist treatment. Vitamin K antagonists exert anticoagulant effects by blocking carboxylation of glutamic acid residues of the Vitamin K-dependent coagulation factors during hepatic synthesis, lowering both factor synthesis and function. The administration of KCENTRA rapidly increases plasma levels of the Vitamin K-dependent coagulation Factors II, VII, IX, and X as well as the antithrombotic Proteins C and S.
Coagulation Factor II
Factor II (prothrombin) is converted to thrombin by activated FX (FXa) in the presence of Ca 2+, FV, and phospholipids.
Coagulation Factor VII
Factor VII (proconvertin) is converted to the activated form (FVIIa) by splitting of an internal peptide link. The FVIIa-TF complex activates Factor IX and initiates the primary coagulation pathway by activating FX in the presence of phospholipids and calcium ions.
Coagulation Factor IX
Factor IX (antihemophilic globulin B, or Christmas factor) is activated by the FVIIa-TF complex and by FXIa. Factor IXa in the presence of FVIIIa activates FX to FXa.
Coagulation Factor X
Factor X (Stuart-Prower factor) activation involves the cleavage of a peptide bond by the FVIIIa-Factor IXa complex or the TF-FVIIa complex. Factor Xa forms a complex with activated FV (FVa) that converts prothrombin to thrombin in the presence of phospholipids and calcium ions.
Protein C
Protein C, when activated by thrombin, exerts an antithrombotic effect by inhibiting FVa and FVIIIa leading to a decrease in thrombin formation, and has indirect profibrinolytic activity by inhibiting plasminogen activator inhibitor-1.
Protein S
Protein S exists in a free form (40%) and in a complex with C4b-binding protein (60%). Protein S (free form) functions as a cofactor for activated Protein C in the inactivation of FVa and FVIIIa, leading to antithrombotic activity.
12.2. Pharmacodynamics
International Normalized Ratio (INR)
In the plasma-controlled RCT in acute major bleeding, the INR was determined at varying time points after the start or end of infusion, depending upon study design. The median INR was above 3.0 prior to the infusion and dropped to a median value of 1.20 by the 30 minute time point after start of KCENTRA infusion. By contrast, the median value for plasma was 2.4 at 30 minutes after the start of infusion. The INR differences between KCENTRA and plasma were statistically significant in randomized plasma-controlled trial in bleeding up to 12 hours after start of infusion [see Table 8].
The relationship between these or other INR values and clinical hemostasis in patients has not been established [see Clinical Studies (14)].
Table 8. Median INR (Min-Max) after Start of Infusion in RCTs:
| Study | Treatment | Baseline | 30 min | 1 hr | 2-3 hr | 6-8 hr | 12 hr | 24 hr |
|---|---|---|---|---|---|---|---|---|
| Acute Major Bleeding Study | KCENTRA (N=98) | 3.90 (1.8–20.0) | 1.20* (0.9–6.7) | 1.30* (0.9–5.4) | 1.30* (0.9–2.5) | 1.30* (0.9–2.1) | 1.20* (0.9–2.2) | 1.20 (0.9–3.8) |
| Plasma (N=104) | 3.60 (1.9–38.9) | 2.4 (1.4–11.4) | 2.1 (1.0–11.4) | 1.7 (1.1–4.1) | 1.5 (1.0–3.0) | 1.4 (1.0–3.0) | 1.3 (1.0–2.9) | |
| Urgent Surgery/ Invasive Procedures Study | KCENTRA (N=87) | 2.90 (2.0–17.0) | 1.30* (0.9–7.0) | 1.20* (0.9–2.5) | 1.30* (0.9–39.2) | 1.30* (1.0–10.3) | NC | 1.20 (0.9–2.7) |
| Plasma (N=81) | 2.90 (2.0–26.7) | 2.15 (1.4–5.4) | 1.90 (1.3–5.7) | 1.70 (1.1–3.7) | 1.60 (1.0–5.8) | NC | 1.30 (1.0–2.7) |
INR = international normalized ratio; NC = not collected.
* Statistically significant difference compared to plasma by 2-sided Wilcoxon test
12.3. Pharmacokinetics
Fifteen healthy subjects received 50 units/kg of KCENTRA. No subjects were receiving VKA therapy or were experiencing acute bleeding. A single intravenous KCENTRA infusion produced a rapid and sustained increase in plasma concentration of Factors II, VII, IX and X as well as Proteins C and S. The PK analysis [see Table 9] shows that factor II had the longest half-life (59.7 hours) and factor VII the shortest (4.2 hours) in healthy subjects. PK parameters obtained from data derived from the study of healthy subjects may not be directly applicable to patients with INR elevation due to VKA anticoagulation therapy.
Table 9. Vitamin K-Dependent Coagulation Factor Pharmacokinetics after a Single KCENTRA Infusion in Healthy Subjects (n=15) Mean (SD)*:
| Parameter | Factor IX | Factor II | Factor VII | Factor X | Protein C | Protein S |
|---|---|---|---|---|---|---|
| Terminal half-life (h) | 42.4 (41.6) | 60.4 (25.5) | 5.0 (1.9) | 31.8 (8.7) | 49.6 (32.7) | 50.4 (13.4) |
| IVR (%/units/kg bw)* | 1.6 (0.4) | 2.2 (0.3) | 2.5 (0.4) | 2.2 (0.4) | 2.9 (0.3) | 2.0 (0.3) |
| AUC (IU/dL × h) | 1850.8 (1001.4) | 7282.2 (2324.9) | 512.9 (250.1) | 6921.5 (1730.5) | 5397.5 (2613.9) | 3651.6 (916.3) |
| Clearance (mL/ kg × h) | 3.7 (1.6) | 1.0 (0.3) | 7.4 (4.1) | 1.3 (0.3) | 1.5 (0.9) | 1.2 (0.3) |
| MRT (h)† | 47.3 (49.5) | 82.0 (34.2) | 7.1 (2.7) | 45.9 (12.6) | 62.4 (42.1) | 70.3 (18.3) |
| Vdss (mL/kg)‡ | 114.3 (54.6) | 71.4 (13.7) | 45.0 (10.7) | 55.5 (6.7) | 62.2 (17.4) | 78.8 (11.6) |
* IVR: In Vivo Recovery
† MRT: Mean Residence Time
‡ Vdss: Volume of Distribution at steady state
The mean in vivo recovery (IVR) of infused factors was calculated in subjects who received KCENTRA. The IVR is the increase in measurable factor levels in plasma (units/dL) that may be expected following an infusion of factors (units/kg) administered as a dose of KCENTRA. The in vivo recovery ranged from 1.15 (Factor IX) to 2.81 (Protein S) [see Table 10].
Table 10. In vivo Recovery in RCTs*:
| Parameter | Incremental (units/dL per units/kg b.w.) | |||
|---|---|---|---|---|
| Acute Major Bleeding Study (N=98) | Urgent Surgery/Invasive Procedures Study (N=87) | |||
| Mean (SD) | 95% CI† | Mean (SD) | 95% CI† | |
| Factor IX | 1.29 (0.71) | (1.14–1.43) | 1.15 (0.57) | (1.03–1.28) |
| Factor II | 2.00 (0.88) | (1.82–2.18) | 2.14 (0.74) | (1.98–2.31) |
| Factor VII | 2.15 (2.96) | (1.55–2.75) | 1.90 (4.50) | (0.92–2.88) |
| Factor X | 1.96 (0.87) | (1.79–2.14) | 1.94 (0.69) | (1.79–2.09) |
| Protein C | 2.04 (0.96) | (1.85–2.23) | 1.88 (0.68) | (1.73–2.02) |
| Protein S | 2.17 (1.66) | (1.83–2.50) | 2.81 (1.95) | (2.38–3.23) |
* ITT-E: Intention to Treat – Efficacy Population
† CI: Confidence Interval
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies in animals to evaluate the carcinogenic potential of KCENTRA, or studies to determine the effects of KCENTRA on genotoxicity or fertility have not been performed. An assessment of the carcinogenic potential of KCENTRA was completed and suggests minimal carcinogenic risk from product use.
14. Clinical Studies
Acute Major Bleeding RCT
The efficacy of KCENTRA has been evaluated in a prospective, open-label, (blinded assessor), active-controlled, non-inferiority, multicenter RCT in subjects who had been treated with VKA therapy and who required urgent replacement of their Vitamin K-dependent clotting factors to treat acute major bleeding. A total of 216 subjects with acquired coagulation factor deficiency due to oral Vitamin K antagonist therapy were randomized to a single dose of KCENTRA or plasma. Two hundred twelve (212) subjects received KCENTRA or plasma for acute major bleeding in the setting of a baseline INR ≥ 2.0 and recent use of a VKA anticoagulant. The doses of KCENTRA (25 units/kg, 35 units/kg, or 50 units/kg) based on nominal Factor IX content and plasma (10 mL/kg, 12 mL/kg, or 15 mL/kg) were calculated according to the subject’s baseline INR (2– <4, 4–6, >6, respectively). The observation period lasted for 90 days after the infusion of KCENTRA or plasma. The modified efficacy (ITT-E) population for KCENTRA included 98 subjects and for plasma included 104 subjects. Additionally, intravenous Vitamin K was administered.
The efficacy endpoint was hemostatic efficacy for the time period from the start of infusion of KCENTRA or plasma until 24 hours. Efficacy was adjudicated as “effective” or “not effective” by a blinded, independent Endpoint Adjudication Board for all subjects who received study product. Criteria for effective hemostasis were based upon standard clinical assessments including vital signs, hemoglobin measurements, and CT assessments at pre-defined time points, as relevant to the type of bleeding (i.e., gastrointestinal, intracranial hemorrhage, visible, musculoskeletal, etc.). The proportion of subjects with effective hemostasis was 72.4% in the KCENTRA group and 65.4% in the plasma group. The lower limit of the 95% confidence interval (CI) for the difference in proportions of KCENTRA minus plasma was -5.8%, which exceeded -10% and thereby demonstrated the non-inferiority of KCENTRA versus plasma (the study’s primary objective) [see Table 11]. Because the lower limit of the CI was not greater than zero, the prospectively defined criterion for superiority of KCENTRA for hemostatic efficacy (a secondary objective) was not met.
Table 11. Rating of Hemostatic Efficacy in Subjects with Acute Major Bleeding:
| Rating | No. (%) of subjects [95% CI] | Difference KCENTRA – Plasma (%) [95% CI]* | |
|---|---|---|---|
| KCENTRA (N=98) | Plasma (N=104) | ||
| “Effective” hemostasis | 71 (72.4%) [62.3; 82.6] | 68 (65.4%) [54.9; 75.8] | [-5.8; 19.9] |
CI = confidence interval; N = number of subjects
* KCENTRA non-inferior to plasma if lower limit of 95% CI > –10%;
KCENTRA superior to plasma if lower limit of 95% CI > 0.
Results of a post-hoc analysis of hemostatic efficacy stratified by actual dose of KCENTRA or plasma administered in the acute major bleeding RCT are presented in Table 12.
Table 12. Rating of Hemostatic Efficacy Stratified by Actual Dose of KCENTRA or Plasma (Number and % of Subjects rated “Effective”) in Acute Major Bleeding RCT:
| Low Dose | Mid Dose | High Dose | |
|---|---|---|---|
| N=49 (K) | N=22 (K) | N=26 (K) | |
| N=55 (P) | N=18 (P) | N=31 (P) | |
| KCENTRA | 36 (74.5%) | 16 (72.7%) | 18 (69.2%) |
| Plasma | 38 (69.1%) | 11 (61.1%) | 19 (61.3%) |
| Difference* | (4.4%) | (11.6%) | (7.9%) |
| 95% CI K–P | -13.2–21.9 | -17.4–40.6 | -17.0–32.9 |
* KCENTRA minus Plasma
An additional endpoint was the reduction of INR to ≤ 1.3 at 30 minutes after the end of infusion of KCENTRA or plasma for all subjects that received study product. The proportion of subjects with this decrease in INR was 62.2% in the KCENTRA group and 9.6% in the plasma group. The 95% confidence interval for the difference in proportions of KCENTRA minus plasma was 39.4% to 65.9%. The lower limit of the 95% CI of 39.4% demonstrated superiority of KCENTRA versus plasma for this endpoint [see Table 13].
Table 13. Decrease of INR (1.3 or Less at 30 Minutes after End of Infusion) in Acute Major Bleeding RCT:
| Rating | No. (%) of subjects [95% CI] | Difference KCENTRA – Plasma (%) [95% CI]* | |
|---|---|---|---|
| KCENTRA (N=98) | Plasma (N=104) | ||
| Decrease of INR to ≤ 1.3 at 30 min | 61 (62.2%) [52.6; 71.8] | 10 (9.6%) [3.9; 15.3] | (52.6%) [39.4; 65.9] |
CI = confidence interval; INR = international normalized ratio; N = total subjects
* KCENTRA non-inferior to plasma if lower limit of 95% CI > –10%; KCENTRA superior to plasma if lower limit of 95% CI > 0.
Urgent Surgery/Invasive Procedure RCT
The efficacy of KCENTRA has been evaluated in a prospective, open-label, active-controlled, non-inferiority, multicenter RCT in subjects who had been treated with VKA therapy and who required urgent replacement of their Vitamin K-dependent clotting factors because of their need for an urgent surgery/invasive procedure. A total of 181 subjects with acquired coagulation factor deficiency due to oral Vitamin K antagonist therapy were randomized to a single dose of KCENTRA or plasma. One hundred seventy-six (176) subjects received KCENTRA or plasma because of their need for an urgent surgery/invasive procedure in the setting of a baseline INR ≥ 2.0 and recent use of a VKA anticoagulant. The doses of KCENTRA (25 units/kg, 35 units/kg, or 50 units/kg) based on nominal Factor IX content and plasma (10 mL/kg, 12 mL/kg, or 15 mL/kg) were calculated according to the subject’s baseline INR (2– <4, 4–6, >6, respectively). The observation period lasted for 90 days after the infusion of KCENTRA or plasma. The modified efficacy (ITT-E) population for KCENTRA included 87 subjects and for plasma included 81 subjects. Additionally, oral or intravenous Vitamin K was administered.
The efficacy endpoint was hemostatic efficacy for the time period from the start of infusion of KCENTRA or plasma until the end of the urgent surgery/invasive procedure. Criteria for effective hemostasis were based upon the difference between predicted and actual blood losses, subjective hemostasis rating, and the need for additional blood products containing coagulation factors. The proportion of subjects with effective hemostasis was 89.7% in the KCENTRA group and 75.3% in the plasma group. The lower limit of the 95% confidence interval (CI) for the difference in proportions of KCENTRA minus plasma was 2.8%, which exceeded -10% and thereby demonstrated the non-inferiority of KCENTRA versus plasma (the study’s primary objective) [see Table 14]. Because the lower limit of the CI was greater than 0, the prospectively defined criterion for superiority of KCENTRA for hemostatic efficacy (a secondary objective) was also met.
Table 14. Rating of Hemostatic Efficacy in Urgent Surgery/Invasive Procedure RCT:
| Rating | No. (%) of subjects [95% CI] | Difference KCENTRA – Plasma (%) [95% CI]* | |
|---|---|---|---|
| KCENTRA (N=87) | Plasma (N=81) | ||
| “Effective” hemostasis | 78 (89.7%) [83.3; 96.1] | 61 (75.3%) [65.9; 84.7] | (14.3%) [2.8; 25.8] |
CI = confidence interval; N = number of subjects
* KCENTRA non-inferior to plasma if lower limit of 95% CI > –10%; KCENTRA superior to plasma if lower limit of 95% CI > 0.
Results of a post-hoc analysis of hemostatic efficacy stratified by actual dose of KCENTRA or plasma administered in the urgent surgery/invasive procedure RCT are presented in Table 15.
Table 15. Rating of Hemostatic Efficacy Stratified by Actual Dose of KCENTRA or Plasma (Number and % of Subjects rated “Effective”) in Urgent Surgery/Invasive Procedure RCT:
| Low Dose | Mid Dose | High Dose | |
|---|---|---|---|
| N=69 (K) | N=10 (K) | N=8 (K) | |
| N=62 (P) | N=10 (P) | N=9 (P) | |
| KCENTRA | 63 (91.3%) | 8 (80.0%) | 7 (87.5%) |
| Plasma | 48 (77.4%) | 7 (70.0%) | 6 (66.7%) |
| Difference* | (13.9%) | (10.0%) | (20.8%) |
| 95% CI K–P | 1.4–26.6 | -26.5–43.5 | -19.8–53.7 |
* KCENTRA minus Plasma
An additional endpoint was the reduction of INR to ≤ 1.3 at 30 minutes after the end of infusion of KCENTRA or plasma for all subjects that received study product. The proportion of subjects with this decrease in INR was 55.2% in the KCENTRA group and 9.9% in the plasma group. The 95% confidence interval for the difference in proportions of KCENTRA minus plasma was 31.9% to 56.4%. The lower limit of the 95% CI of 31.9% demonstrated superiority of KCENTRA versus plasma for this endpoint [see Table 16]. The relationship between a decrease in INR to less than or equal to 1.3 and clinical hemostatic efficacy has not been established.
Table 16. Decrease of INR (1.3 or Less at 30 Minutes after End of Infusion) in Urgent Surgery/Invasive Procedure RCT:
| Rating | No. (%) of subjects [95% CI] | Difference KCENTRA – Plasma (%) [95% CI]* | |
|---|---|---|---|
| KCENTRA (N=87) | Plasma (N=81) | ||
| Decrease of INR to ≤ 1.3 at 30 min | 48 (55.2%) [44.7; 65.6] | 8 (9.9%) [3.4; 16.4] | (45.3%) [31.9; 56.4] |
CI = confidence interval; INR = international normalized ratio; N = total subjects
The European Bleeding and Surgical Study was an open-label, single-arm, multicenter study. 1 Forty-three (43) subjects who were receiving VKA were treated with KCENTRA, because they either (1) required a surgical or an invasive diagnostic intervention (26 subjects), or (2) experienced an acute bleeding event (17 subjects). The dose of KCENTRA (25 units/kg, 35 units/kg, or 50 units/kg) based on nominal Factor IX content was calculated according to the subject’s baseline INR value (2 – <4, 4–6, >6). The endpoint was the decrease of the INR to ≤ 1.3 within 30 minutes after end of KCENTRA infusion in subjects who received any portion of study product.
Of the 17 evaluable subjects receiving KCENTRA for acute bleeding, 16 subjects (94%) experienced a decrease in INR to ≤ 1.3 within 30 minutes after the end of the KCENTRA infusion.
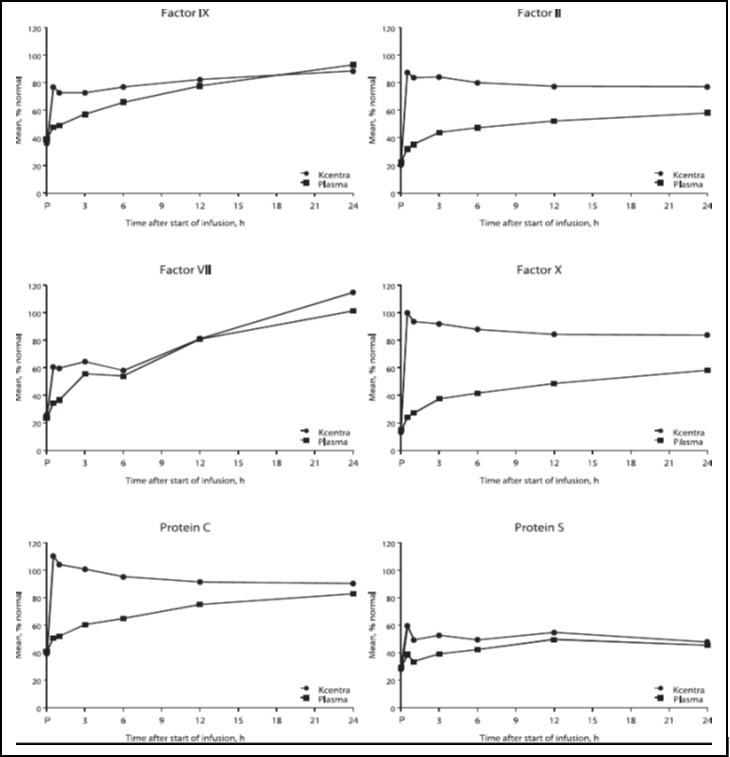
In RCTs, levels of Coagulation Factors II, VII, IX, X, and Antithrombotic Proteins C and S were measured after the infusion of KCENTRA or plasma and the results were similar for subjects with acute major bleeding or subjects requiring an urgent surgery or invasive procedure. In the plasma-controlled RCT in acute major bleeding, the mean duration of KCENTRA infusion was 24 minutes (± 32 minutes) and the mean duration of infusion for plasma was 169 minutes (± 143 minutes). The mean infusion volume of KCENTRA was 105 mL ± 37 mL and the mean infusion volume of plasma was 865 mL ± 269 mL. In the plasma-controlled RCT for patients needing urgent surgery/invasive procedures, the mean duration of KCENTRA infusion was 21 minutes (± 14 minutes) and the mean duration of infusion for plasma was 141 minutes (± 113 minutes). The mean infusion volume of KCENTRA was 90 mL ± 32 mL and the mean infusion volume of plasma was 819 mL ± 231 mL.
The increase in mean factor levels over time following KCENTRA and plasma administration in the plasma-controlled RCT in acute major bleeding is shown in Figure 9 below (the mean factor levels over time following KCENTRA and plasma administration in the plasma-controlled RCT for patients needing urgent surgery/invasive procedures are not shown, but showed similar profiles). Levels of some factors continued to increase at later time points, consistent with the effect of concomitant Vitamin K treatment. Formal pharmacokinetic parameters were not derived because of the effect of Vitamin K on factor levels at time points required for pharmacokinetic profiling.
Figure 9. Mean Factor Levels (Factors II, VII, IX, X, Proteins C & S) over 24 hours in Acute Major Bleeding RCT:
Time axis is scheduled measuring time: hours after start of infusion (P=pre-infusion)
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.