KERYDIN Topical solution Ref.[10215] Active ingredients: Tavaborole
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
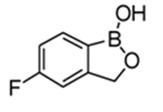
KERYDIN (tavaborole) topical solution, 5% contains tavaborole, 5% (w/w) in a clear, colorless alcohol-based solution for topical use. The active ingredient, tavaborole, is an oxaborole antifungal with the chemical name of 5-fluoro-1,3-dihydro-1-hydroxy-2,1-benzoxaborole. The chemical formula is C7H6BFO2, the molecular weight is 151.93 and the structural formula is:
Tavaborole is a white to off-white powder. It is slightly soluble in water and freely soluble in ethanol and propylene glycol.
Each mL of KERYDIN contains 43.5 mg of tavaborole. Inactive ingredients include alcohol, edetate calcium disodium, and propylene glycol.
| Dosage Forms and Strengths |
|---|
|
KERYDIN topical solution, 5% is a clear, colorless alcohol-based solution. Each milliliter of solution contains 43.5 mg (5% w/w) of tavaborole. |
| How Supplied |
|---|
|
KERYDIN (tavaborole) topical solution, 5% is a clear, colorless solution supplied in an amber glass bottle with a screw cap. At initial use, the screw cap is replaced with the dropper assembly. KERYDIN (tavaborole) topical solution, 5% is supplied in the following presentations: NDC 10337-905-10: One bottle containing 10 mL of solution with one glass pointed-tip dropper. NDC 10337-905-44: One bottle containing 4 mL of solution with one glass pointed-tip dropper. |
Drugs
| Drug | Countries | |
|---|---|---|
| KERYDIN | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.