KEVEYIS Tablet Ref.[10013] Active ingredients: Diclofenamide
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
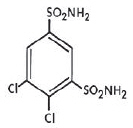
KEVEYIS (dichlorphenamide) tablets is an oral carbonic anhydrase inhibitor. Dichlorphenamide, a dichlorinated benzenedisulfonamide, is known chemically as 4, 5–dichloro-1,3-benzenedisulfonamide.
Its empirical formula is C6H6Cl2N2O4S2 and its structural formula is:
Dichlorphenamide USP is a white or practically white, crystalline compound with a molecular weight of 305.16. It is very slightly soluble in water but soluble in dilute solutions of sodium carbonate and sodium hydroxide. Dilute alkaline solutions of dichlorphenamide are stable at room temperature.
KEVEYIS (dichlorphenamide) tablets is supplied as tablets, for oral administration, each containing 50 mg dichlorphenamide. Inactive ingredients are lactose monohydrate, magnesium stearate and pregelatinized maize starch.
| Dosage Forms and Strengths |
|---|
|
Round, white tablets, scored on one side, engraved with “TARO” on one side and on the other side “D” above the score and “50” below the score, 50 mg each. |
| How Supplied | ||
|---|---|---|
|
Each KEVEYIS (dichlorphenamide) tablets, 50 mg – round, white tablet, scored on one side, engraved with “TARO” on one side and on the other side “D” above the score and “50” below the score. KEVEYIS (dichlorphenamide) tablets are supplied as follows:
|
Drugs
| Drug | Countries | |
|---|---|---|
| KEVEYIS | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.