KEVZARA 150 mg / 200 mg Solution for injection Ref.[8831] Active ingredients: Sarilumab
Source: European Medicines Agency (EU) Revision Year: 2025 Publisher: Sanofi Winthrop Industrie, 82 avenue Raspail, 94250 Gentilly, France
Pharmacodynamic properties
Pharmacotherapeutic group: Immunosuppressants, Interleukin inhibitors
ATC code: L04AC14
Mechanism of action
Sarilumab is a human monoclonal antibody (IgG1 subtype) that specifically binds to both soluble and membrane-bound IL-6 receptors (IL-6Rα), and inhibits IL-6-mediated signalling which involves ubiquitous signal-transducing glycoprotein 130 (gp130) and the Signal Transducer and Activator of Transcription-3 (STAT-3).
In functional human cell-based assays, sarilumab was able to block the IL-6 signalling pathway, measured as STAT-3 inhibition, only in the presence of IL-6.
IL-6 is a pleiotropic cytokine that stimulates diverse cellular responses such as proliferation, differentiation, survival, and apoptosis and can activate hepatocytes to release acute-phase proteins, including C-reactive protein (CRP) and serum amyloid A. Elevated levels of IL-6 are found in the synovial fluid of patients with rheumatoid arthritis (RA) and polyarticular juvenile idiopathic arthritis (pJIA) and play an important role in both the pathologic inflammation and joint destruction which are hallmarks of RA and pJIA. IL-6 is involved in diverse physiological processes such as migration and activation of T-cells, B-cells, monocytes, and osteoclasts leading to systemic inflammation, synovial inflammation, and bone erosion in patients with RA and pJIA.
The activity of sarilumab in reducing inflammation is associated with laboratory changes such as decrease in ANC and elevation in lipids (see section 4.4).
Pharmacodynamic effects
Following single-dose subcutaneous (SC) administration of sarilumab 200 mg and 150 mg in patients with RA rapid reduction of CRP levels was observed. Levels were reduced to normal as early as 4 days after treatment initiation. Following single-dose sarilumab administration, in patients with RA, ANC decreased to the nadir between 3 to 4 days and thereafter recovered towards baseline (see section 4.4). Treatment with sarilumab resulted in decreases in fibrinogen and serum amyloid A, and increases in haemoglobin and serum albumin. Sarilumab treatment for PMR patients taking 200 mg once every 2 weeks has a similar effect compared to RA patients on the PD biomarker profiles (CRP and ANC) over time.
Clinical efficacy
Rheumatoid arthritis (RA)
The efficacy and safety of sarilumab were assessed in three randomised, double-blind, controlled multicentre studies (MOBILITY and TARGET were placebo-controlled studies and MONARCH was an active comparator-controlled study) in patients older than 18 years with moderately to severely active rheumatoid arthritis diagnosed according to American College of Rheumatology (ACR) criteria. Patients had at least 8 tender and 6 swollen joints at baseline.
Placebo-controlled studies
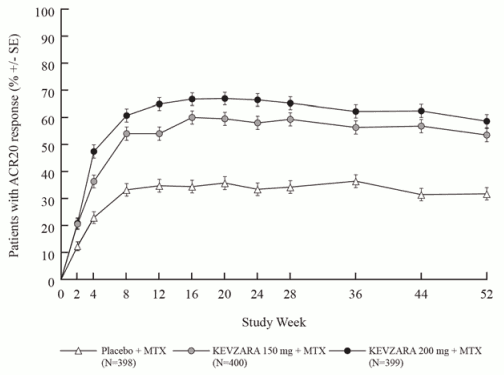
MOBILITY evaluated 1197 patients with RA who had inadequate clinical response to MTX. Patients received sarilumab 200 mg, sarilumab 150 mg, or placebo every 2 weeks with concomitant MTX. The primary endpoints were the proportion of patients who achieved an ACR20 response at week 24, changes from baseline in Health Assessment Questionnaire – Disability Index (HAQ-DI) score at week 16, and change from baseline in van der Heijde-modified Total Sharp Score (mTSS) at week 52.
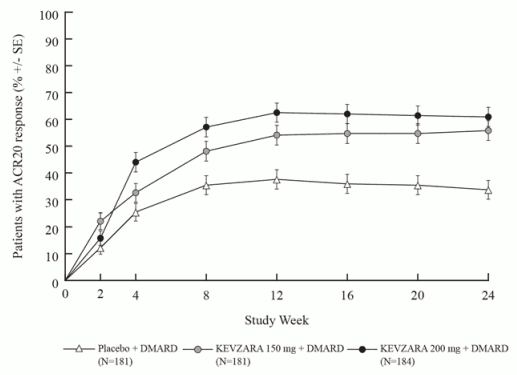
TARGET evaluated 546 patients with RA who had an inadequate clinical response or were intolerant to one or more TNF-α antagonists. Patients received sarilumab 200 mg, sarilumab 150 mg, or placebo every 2 weeks with concomitant conventional DMARDs (cDMARDs). The primary endpoints were the proportion of patients who achieved an ACR20 response at week 24 and the changes from baseline HAQ-DI score at week 12.
Clinical response
The percentages of sarilumab + DMARDs-treated patients achieving ACR20, ACR50, and ACR70 responses in MOBILITY and TARGET are shown in Table 4. In both studies, patients treated with either 200 mg or 150 mg of sarilumab + DMARDs every two weeks had higher ACR20, ACR50, and ACR70 response rates versus placebo-treated patients at week 24. These responses persisted through 3 years of therapy in an open-label extension study.
In MOBILITY, a greater proportion of patients treated with sarilumab 200 mg or 150 mg every two weeks plus MTX achieved remission, defined as Disease Activity Score 28-C-Reactive Protein (DAS28-CRP) <2.6 compared with placebo + MTX at week 52. Results at 24 weeks in TARGET were similar to the results at 52 weeks in MOBILITY (see Table 4).
Table 4. Clinical response at weeks 12, 24, and 52 in placebo-controlled studies, MOBILITY and TARGET:
| Percentage of patients | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MOBILITY MTX inadequate responders | TARGET TNF inhibitor inadequate responders | ||||||||||||
| Placebo + MTX N=398 | Sarilumab 150 mg + MTX N=400 | Sarilumab 200 mg + MTX N=399 | Placebo + cDMARDs* N=181 | Sarilumab 150 mg + cDMARDs* N=181 | Sarilumab 200 mg + cDMARDs* N=184 | ||||||||
| Week 12 | |||||||||||||
| DAS28-CRP remission (<2.6) | 4.8% | 18.0%††† | 23.1%††† | 3.9% | 17.1%††† | 17.9%††† | ACR20 | 34.7% | 54.0%††† | 64.9%††† | 37.6% | 54.1%† | 62.5%††† |
| ACR50 | 12.3% | 26.5%††† | 36.3%††† | 13.3% | 30.4%††† | 33.2%^††† | |||||||
| ACR70 | 4.0% | 11.0%^†† | 17.5%††† | 2.2% | 13.8%††† | 14.7%††† | |||||||
| Week 24 | |||||||||||||
| DAS28-CRP remission (<2.6) | 10.1% | 27.8%††† | 34.1%††† | 7.2% | 24.9%††† | 28.8%††† | |||||||
| ACR20‡ | 33.4% | 58.0%††† | 66.4%††† | 33.7% | 55.8%††† | 60.9%††† | |||||||
| ACR50 | 16.6% | 37.0%††† | 45.6%††† | 18.2% | 37.0%††† | 40.8%††† | |||||||
| ACR70 | 7.3% | 19.8%††† | 24.8%††† | 7.2% | 19.9%†† | 16.3%† | |||||||
| Week 52 | |||||||||||||
| DAS28-CRP remission (<2.6) | 8.5% | 31.0%††† | 34.1%††† | NA§ | NA§ | NA§ | |||||||
| ACR20 | 31.7% | 53.5%††† | 58.6%††† | ||||||||||
| ACR50 | 18.1% | 40.0%††† | 42.9%††† | ||||||||||
| ACR70 | 9.0% | 24.8% | 26.8% | ||||||||||
| Major clinical response¶ | 3.0% | 12.8%††† | 14.8%††† | ||||||||||
*cDMARDs in TARGET included MTX, sulfasalazine, leflunomide and hydroxychloroquine
† p-value<0.01 for difference from placebo
†† p-value<0.001 for difference from placebo
††† p-value<0.0001 for difference from placebo
‡ Primary endpoint
§ NA=Not Applicable as TARGET was a 24-week study
¶ Major clinical response = ACR70 for at least 24 consecutive weeks during the 52-week period
In both MOBILITY and TARGET, higher ACR20 response rates were observed within 2 weeks compared to placebo and were maintained for the duration of the studies (see Figures 1 and 2).
Figure 1. Percent of ACR20 response by visit for MOBILITY:
Figure 2. Percent of ACR20 response by visit for TARGET:
The results of the components of the ACR response criteria at week 24 for MOBILITY and TARGET are shown in Table 5. Results at 52 weeks in MOBILITY were similar to the results at 24weeks for TARGET.
Table 5. Mean reductions from baseline to week 24 in components of ACR score:
| MOBILITY | TARGET | |||||
|---|---|---|---|---|---|---|
| Component (range) | Placebo + MTX (N=398) | Sarilumab 150 mg q2w* + MTX (N=400) | Sarilumab 200 mg q2w* + MTX (N=399) | Placebo + cDMARDs (N=181) | Sarilumab 150 mg q2w* + cDMARDs (N=181) | Sarilumab 200 mg q2w* + cDMARDs (N=184) |
| Tender Joints (0-68) | -14.38 | -19.25††† | -19.00††† | -17.18 | -17.30† | -20.58††† |
| Swollen Joints (0-66) | -8.70 | -11.84††† | -12.43††† | -12.12 | -13.04†† | -14.03††† |
| Pain VAS† (0-100 mm) | -19.43 | -30.75††† | -34.35††† | -27.65 | -36.28†† | -39.60††† |
| Physician global VAS‡ (0-100 mm) | -32.04 | -40.69††† | -42.65††† | -39.44 | -45.09††† | -48.08††† |
| Patient global VAS‡ (0-100 mm) | -19.55 | -30.41††† | -35.07††† | -28.06 | -33.88†† | -37.36††† |
| HAQ-DI (0-3) | -0.43 | -0.62††† | -0.64††† | -0.52 | -0.60† | -0.69†† |
| CRP | -0.14 | -13.63††† | -18.04††† | -5.21 | -13.11††† | -29.06††† |
* q2w = every 2 weeks
‡ Visual analogue scale
† p-value<0.01 for difference from placebo
†† p-value<0.001 for difference from placebo
††† p-value<0.0001 for difference from placebo
Radiographic response
In MOBILITY, structural joint damage was assessed radiographically and expressed as change in van der Heijde-modified Total Sharp Score (mTSS) and its components, the erosion score, and joint space narrowing score at week 52. Radiographs of hands and feet were obtained at baseline, 24 weeks, and 52 weeks and scored independently by at least two well-trained readers who were blinded to treatment group and visit number.
Both doses of sarilumab + MTX were superior to placebo + MTX in the change from baseline in mTSS at 24 and 52 weeks (see Table 6). Less progression of both erosion and joint space narrowing scores at 24 and 52 weeks was reported in the sarilumab treatment groups compared to the placebo group.
Treatment with sarilumab + MTX was associated with significantly less radiographic progression of structural damage as compared with placebo. At week 52, 55.6% of patients receiving sarilumab 200 mg and 47.8% of patients receiving sarilumab 150 mg had no progression of structural damage (as defined by a change in the TSS of zero or less) compared with 38.7% of patients receiving placebo.
Treatment with sarilumab 200 mg and 150 mg + MTX inhibited the progression of structural damage by 91% and 68%, respectively, compared to placebo + MTX at week 52.
The efficacy of sarilumab with concomitant DMARDs on inhibition of radiographic progression that was assessed as part of the primary endpoints at week 52 in MOBILITY was sustained up to three years from the start of treatment.
Table 6. Mean radiographic change from baseline at week 24 and week 52 in MOBILITY:
| MOBILITY MTX Inadequate responders | |||
|---|---|---|---|
| Placebo + MTX (N=398) | Sarilumab 150 mg q2w* + MTX (N=400) | Sarilumab 200 mg q2w* + MTX (N=399) | |
| Mean change at week 24 | |||
| Modified Total Sharp Score (mTSS) | 1.22 | 0.54† | 0.13†† |
| Erosion score (0-280) | 0.68 | 0.26† | 0.02†† |
| Joint space narrowing score | 0.54 | 0.28 | 0.12† |
| Mean change at week 52 | |||
| Modified Total Sharp Score (mTSS)‡ | 2.78 | 0.90†† | 0.25†† |
| Erosion score (0-280) | 1.46 | 0.42†† | 0.05†† |
| Joint space narrowing score | 1.32 | 0.47† | 0.20†† |
* q2w = every two weeks
† p-value<0.001
†† p-value<0.0001
‡ Primary end point
Physical function response
In MOBILITY and TARGET, physical function and disability were assessed by the Health Assessment Questionnaire Disability Index (HAQ-DI). Patients receiving sarilumab 200 mg or 150 mg + DMARDs every two weeks demonstrated greater improvement from baseline in physical function compared to placebo at week 16 and week 12 in MOBILITY and TARGET, respectively.
MOBILITY demonstrated significant improvement in physical function, as measured by the HAQ-DI at week 16 compared to placebo (-0.58, -0.54, and -0.30 for sarilumab 200 mg + MTX, sarilumab 150 mg + MTX, and placebo + MTX, every two weeks, respectively). TARGET demonstrated significant improvement in HAQ-DI scores at week 12 compared to placebo (-0.49, -0.50, and -0.29 for sarilumab 200 mg + DMARDs, sarilumab 150 mg + DMARDs, and placebo + DMARDs, every two weeks, respectively).
In MOBILITY, the improvement in physical functioning as measured by HAQ-DI was maintained up to week 52 (-0.75, -0.71, and -0.46 for sarilumab 200 mg + MTX, sarilumab 150 mg + MTX, and placebo + MTX treatment groups, respectively).
Patients treated with sarilumab + MTX (47.6% in the 200 mg treatment group and 47.0% in the 150 mg treatment group) achieved a clinically relevant improvement in HAQ-DI (change from baseline of ≥0.3 units) at week 52 compared to 26.1% in the placebo + MTX treatment group.
Patient reported outcomes
General health status was assessed by the Short Form health survey (SF-36). In MOBILITY and TARGET, patients receiving sarilumab 200 mg + DMARDs every two weeks or sarilumab 150 mg + DMARDs every two weeks demonstrated greater improvement from baseline compared to placebo + DMARDs in physical component summary (PCS) and no worsening on the mental component summary (MCS) at week 24. Patients receiving sarilumab 200 mg + DMARDs reported greater improvement relative to placebo in the domains of Physical Functioning, Role Physical, Bodily Pain, General Health Perception, Vitality, Social Functioning, and Mental Health.
Fatigue was assessed by the FACIT-Fatigue scale. In MOBILITY and TARGET, patients receiving sarilumab 200 mg + DMARDs every two weeks or sarilumab 150 mg + DMARDs every two weeks demonstrated greater improvement from baseline compared to placebo + DMARDs.
Active Comparator-controlled Study
MONARCH was a 24–week randomised double-blind, double-dummy study that compared sarilumab 200 mg monotherapy with adalimumab 40 mg monotherapy administered subcutaneously every two weeks in 369 patients with moderately to severely active RA who were inappropriate for treatment with MTX including those who were intolerant of or inadequate responders to MTX.
Sarilumab 200 mg was superior to adalimumab 40 mg in reducing disease activity and improving physical function, with more patients achieving clinical remission over 24weeks (see Table 7).
Table 7. Efficacy results for MONARCH:
| Adalimumab 40 mg q2w* (N=185) | Sarilumab 200 mg q2w (N=184) | |
|---|---|---|
| DAS28-ESR (primary endpoint) p-value versus adalimumab | -2.20 (0.106) | -3.28 (0.105) <0.0001 |
| DAS28-ESR remission (<2.6), n (%) p-value versus adalimumab | 13 (7.0%) | 49 (26.6%) <0.0001 |
| ACR20 response, n (%) p-value versus adalimumab | 108 (58.4%) | 132 (71.7%) 0.0074 |
| ACR50 response, n (%) p-value versus adalimumab | 55 (29.7%) | 84 (45.7%) 0.0017 |
| ACR70 response, n (%) p-value versus adalimumab | 22 (11.9%) | 43 (23.4%) 0.0036 |
| HAQ-DI p-value versus adalimumab | -0.43(0.045) | -0.61(0.045) 0.0037 |
* Includes patients who increased the frequency of dosing of adalimumab 40 mg to every week because of an inadequate response
Polymyalgia rheumatica (PMR)
The efficacy and safety of sarilumab were assessed in a randomised, double-blind, placebo-controlled multicentre study (SAPHYR) in patients 50 years and older with PMR, diagnosed according to American College of Rheumatology/European Union League against Rheumatism (ACR/EULAR) classification criteria. Patients had at least one episode of unequivocal PMR flare while attempting to taper corticosteroids.
In the SAPHYR study, patients with active PMR were randomised to receive sarilumab 200 mg every two weeks with a pre-defined 14-week taper of prednisone (n= 60) or placebo every two weeks with a pre-defined 52-week taper of prednisone (n=58). One patient was randomized but not treated in the sarilumab 200 mg arm. The number of patients who completed the study treatment period was 42 (70%) and 36 (62.1%) in the sarilumab group and placebo group, respectively. Patients experiencing a disease flare or unable to adhere to the assigned prednisone tapering schedule could receive corticosteroids as rescue therapy.
By design, the prednisone tapers in the treatment arms differed. The total actual cumulative prednisone equivalent corticosteroid dose in the sarilumab arm (median 777 mg) was lower compared to placebo (median 2044 mg).
The primary end point was the proportion of patients with sustained remission at Week 52. Sustained remission was defined as achievement of disease remission no later than Week 12, absence of disease flare from Week 12 through Week 52, sustained reduction of CRP (to <10 mg/L) from Week 12 through Week 52 and successful adherence to prednisone taper from Week 12 through Week 52. Other endpoints included total cumulative corticosteroid dose over 52 weeks, time to first PMR flare, and patient reported outcomes.
Clinical Response
A greater proportion of patients in the sarilumab arm achieved sustained remission at Week 52 compared to the placebo arm (p=0.0193). At 52 weeks, a higher proportion of patients in the sarilumab arm achieved each component of the sustained remission endpoint compared to placebo. The cumulative corticosteroid dose during the 52-week treatment period was lower in the sarilumab arm compared to placebo (see Table 8).
Table 8. Clinical Response in Adults with Active PMR (SAPHYR study):
| Placebo (N=58) | Sarilumab (N=60) | p value vs placebo | ||
|---|---|---|---|---|
| Sustained remission at Week 52 | ||||
| Number of patients with sustained remission | n (%) | 6 (10.3) | 17 (28.3) | |
| Proportion difference (95% CI) vs. placebo | 18.0 (4.15, 31.82) | 0.0193 | ||
| Components of sustained remission at Week 52 | ||||
| Absence of signs and symptoms and CRP <10 mg/L (disease remission*) no later than Week 12 | n (%) | 22 (37.9) | 28 (46.7) | NC† |
| Absence of disease flare‡ from Week 12 through Week 52 | n (%) | 19 (32.8) | 33 (55.0) | NC |
| Sustained reduction of CRP (<10 mg/L) from Week 12 through Week 52 | n (%) | 26 (44.8) | 40 (66.7) | NC |
| Successful adherence to prednisone taper from Week 12 through Week 52 | n (%) | 14 (24.1) | 30 (50.0) | NC |
* Disease remission is defined as the resolution of signs and symptoms of PMR, and normalization of CRP (<10 mg/L).
† NC: Not calculated
‡ Flare is defined as recurrence of signs and symptoms attributable to active PMR requiring an increase in corticosteroid dose, or elevation of ESR attributable to active PMR plus an increase in corticosteroid dose.
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Kevzara (sarilumab) in all subsets of the paediatric population in polymyalgia rheumatica (see section 4.2 for information on paediatric use).
The European Medicines Agency has deferred the obligation to submit the results of studies with Kevzara (sarilumab) in one or more subsets of the paediatric population in chronic idiopathic arthritis (including rheumatoid arthritis, spondylarthritis, psoriatic arthritis and juvenile idiopathic arthritis) (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Rheumatoid arthritis
The pharmacokinetics of sarilumab were characterised in 2186 adult patients with RA treated with sarilumab which included 751 patients treated with 150 mg and 891 patients treated with 200 mg subcutaneous doses every two weeks for up to 52 weeks.
Absorption
The absolute bioavailability for sarilumab after SC injection was estimated to be 80% by population PK analysis. The median tmax after a single subcutaneous dose was observed in 2 to 4 days. After multiple dosing of 150 to 200 mg every two weeks, steady state was reached in 12 to 16 weeks with a 2- to 3-fold accumulation compared to single dose exposure.
For the 150 mg every two weeks dose regimen, the estimated mean (± standard deviation, SD) steady-state area under curve (AUC), Cmin, and Cmax of sarilumab were 210 ± 115 mg.day/L, 6.95 ± 7.60 mg/L, and 20.4 ± 8.27 mg/L, respectively.
For the 200 mg every two weeks dose regimen, the estimated mean (± SD) steady-state AUC, Cmin and Cmax of sarilumab were 396 ± 194 mg.day/L, 16.7 ± 13.5 mg/L, and 35.4 ± 13.9 mg/L, respectively. In a usability study sarilumab exposure after 200 mg Q2W was slightly higher (Cmax + 24-34%, AUC(0-2w) +7-21%) after use of a pre-filled pen compared to the pre-filled syringe.
Distribution
In patients with RA, the apparent volume of distribution at steady state was 8.3 L.
Biotransformation
The metabolic pathway of sarilumab has not been characterised. As a monoclonal antibody sarilumab is expected to be degraded into small peptides and amino acids via catabolic pathways in the same manner as endogenous IgG.
Elimination
Sarilumab is eliminated by parallel linear and non-linear pathways. At higher concentrations, the elimination is predominantly through the linear, non-saturable proteolytic pathway, while at lower concentrations, non-linear saturable target-mediated elimination predominates. These parallel elimination pathways result in an initial half-life of 8 to 10 days, and at steady-state an effective half-life of 21 days is estimated.
After the last steady state dose of 150 mg and 200 mg sarilumab, the median times to non-detectable concentration are 30 and 49 days, respectively. Monoclonal antibodies are not eliminated via renal or hepatic pathways.
Linearity/non-linearity
A more than dose-proportional increase in pharmacokinetic exposure was observed in patients with RA. At steady state, exposure over the dosing interval measured by AUC increased approximately 2-fold with a 1.33-fold increase in dose from 150 to 200 mg every two weeks.
Interactions with CYP450 substrates
Simvastatin is a CYP3A4 and OATP1B1 substrate. In 17 patients with RA, one week following a single 200-mg subcutaneous administration of sarilumab, exposure of simvastatin and simvastatin acid decreased by 45% and 36%, respectively (see section 4.5).
Polymyalgia rheumatica
The pharmacokinetic characteristics of subcutaneous sarilumab in PMR patients was determined using a population pharmacokinetic analysis including sparse Ctrough observations collected from 58 PMR patients treated with repeated subcutaneous administration of sarilumab 200 mg every two weeks. For this dose regimen, the estimated mean (± SD) steady-state AUC, Cmin and Cmax of sarilumab were 551 ± 321 mg.day/L, 27.0 ± 21.5 mg/L, and 46.5 ± 23.0 mg/L, respectively. PK data analyses suggest the median time to steady state in PMR patients to be approximately 24 weeks. There was accumulation of sarilumab following subcutaneous administration, with an accumulation ratio of 5-6-fold based on the mean trough concentrations.
Special populations
Age, gender, ethnicity and body weight
Population pharmacokinetic analyses in adult patients with RA (ranging in age from 18 to 88 years with 14% over 65 years) showed that age, gender and race did not meaningfully influence the pharmacokinetics of sarilumab.
Body weight influenced the pharmacokinetics of sarilumab in adult patients. In patients with higher body weight (>100 kg) both 150 mg and 200 mg doses demonstrated efficacy; however, patients weighing >100 kg had greater therapeutic benefit with the 200 mg dose.
Renal impairment
No formal study of the effect of renal impairment on the pharmacokinetics of sarilumab was conducted. Mild to moderate renal impairment did not affect the pharmacokinetics of sarilumab. No dose adjustment is required in patients with mild to moderate renal impairment. Patients with severe renal impairment were not studied.
Hepatic impairment
No formal study of the effect of hepatic impairment on the pharmacokinetics of sarilumab was conducted (see section 4.2).
Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of repeated-dose toxicity, carcinogenic risk assessment and toxicity to reproduction and development.
No long-term animal studies have been performed to establish the carcinogenicity potential of sarilumab. The weight of evidence for IL-6Rα inhibition mainly indicates anti-tumour effects mediated by multiple mechanisms predominantly involving STAT-3 inhibition. In vitro and in vivo studies with sarilumab using human tumour cell lines showed inhibition of STAT-3 activation and inhibition of tumour growth in human tumour xenograft animal models.
Fertility studies conducted in male and female mice using a murine surrogate antibody against mouse IL-6Rα showed no impairment of fertility.
In an enhanced pre-/postnatal developmental toxicity study, pregnant Cynomolgus monkeys were administered sarilumab once-weekly intravenously from early gestation to natural birth (approximately 21 weeks) Maternal exposure up to approximately 83 times the human exposure based on AUC after subcutaneous doses of 200 mg every 2 weeks, did not cause any maternal or embryo‑foetal effects. Sarilumab had no effect on maintenance of pregnancy or on the neonates evaluated up to 1 month after birth in body weight measurements, in parameters of functional or morphological development including skeletal evaluations, in immunophenotyping of peripheral blood lymphocytes, and in microscopic evaluations. Sarilumab was detected in the serum of neonates up to 1 month. The excretion of sarilumab in Cynomolgus monkey's milk has not been studied.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.