KINOX Inhalation gas Ref.[27898] Active ingredients: Nitric oxide (NO)
Source: Health Products and Food Branch (CA) Revision Year: 2016
Action and clinical pharmacology
Nitric oxide is a compound produced by many cells of the body. It relaxes vascular smooth muscle by binding to the heme moiety of cytosolic guanylate cyclase, activating guanylate cyclase and increasing intracellular levels of cyclic guanosine 3',5'-monophosphate, which then leads to vasodilation. When inhaled, nitric oxide produces selective pulmonary vasodilation. Nitric oxide is very rapidly inactivated by binding to hemoglobin. Thus, delivered via inhalation, nitric oxide improves V/Q matching and is a selective pulmonary vasodilation agent.
Inhaled nitric oxide appears to increase the partial pressure of arterial oxygen (PaO2) by dilating pulmonary vessels in better ventilated areas of the lung, redistributing pulmonary blood flow away from lung regions with low ventilation/perfusion (V/Q) ratios toward regions with normal ratios.
Effects on Pulmonary Vascular Tone in PPHN: Persistent pulmonary hypertension of the newborn (PPHN) occurs as a primary developmental defect or as a condition secondary to other diseases such as meconium aspiration syndrome (MAS), pneumonia, sepsis, hyaline membrane disease, congenital diaphragmatic hernia (CDH), and pulmonary hypoplasia. In these states, pulmonary vascular resistance (PVR) is high, which results in hypoxemia secondary to right-toleft shunting of blood through the patent ductus arteriosus and foramen ovale. Inhalation of nitric oxide reduces the oxygenation index (OI= mean airway pressure in cm H2O x fraction of inspired oxygen concentration [FiO2] x 100 divided by systemic arterial concentration in mm Hg [PaO2]) and increases PaO2.
Clinical Studies
The efficacy of nitric oxide has been investigated in term and late pre-term newborns with hypoxic respiratory failure, resulting from a variety of etiologies, who had oxygenation index (OI) measurements of ≥25 cm H2O/mm Hg.
In a post-hoc subgroup analysis of data from the NINOS and CINRGI studies, the clinical benefit measured by the receipt of ECMO was greater for the subgroups of patients who did not meet the study ECMO criteria at study entry (NINOS) or whose baseline OI was less than 40 cm H2O/mm Hg (CINRGI).
NINOS study: The Neonatal Inhaled Nitric Oxide Study (NINOS) group conducted a doubleblind, randomized, placebo-controlled, multicenter trial in 235 neonates (≥34 weeks gestational age) with hypoxic respiratory failure and OI values of ≥25 cm H2O/mm Hg.
The objective of the study was to determine whether inhaled nitric oxide would reduce the occurrence of death and/or initiation of extracorporeal membrane oxygenation (ECMO) in a prospectively defined cohort of term or late pre-term neonates with hypoxic respiratory failure unresponsive to conventional therapy. Hypoxic respiratory failure was caused by meconium aspiration syndrome (MAS; 49%), pneumonia/sepsis (21%), idiopathic primary pulmonary hypertension of the newborn (PPHN; 17%), or respiratory distress syndrome (RDS; 11%). Infants up to 14 days of age (mean, 1.7 days) with a mean PaO2 of 46 mm Hg and a mean oxygenation index (OI) of 43 cm H2O/mm Hg were initially randomized to receive 100% O2 with (n=114) or without (n=121) 20 ppm nitric oxide for up to 14 days. Response to study drug was defined as a change from baseline in PaO2 30 minutes after starting treatment (full response = >20 mm Hg, partial = 10-20 mm Hg, no response = <10 mm Hg). Neonates with a less than full response were evaluated for a response to 80 ppm nitric oxide or control gas. The primary results for the intent-to-treat (ITT) population are presented in Table 1.
Table 1. Summary of Clinical Results from NINOS Study ITT Population:
| Control (n=121) | NO (n=114) | P value | Absolute rate reduction (%) | Relative rate reduction (%) | |
|---|---|---|---|---|---|
| Death or ECMOa,b | 77 (64%) | 52 (46%) | 0.006 | -18.0 | -28.3 |
| Death | 20 (17%) | 16 (14%) | 0.60 | Not applicable | Not applicable |
| ECMO | 66 (55%) | 44 (39%) | 0.014 | -15.9 | -29.2 |
a Extracorporeal membrane oxygenation
b Death or need for ECMO was the study's primary end point
No = Nitric Oxide
Although the incidence of death by 120 days of age was similar in both groups (NO, 14%; control, 17%), significantly fewer infants in the nitric oxide group required ECMO compared with controls (39% vs. 55%, p = 0.014). The combined incidence of death and/or initiation of ECMO showed a significant advantage for the nitric oxide treated group (46% vs. 64%, p = 0.006).
The primary efficacy endpoint assessed by the actual gas received was evaluated in a post-hoc analysis and is presented in Table 2.
Table 2. Summary of Clinical Results from NINOS Study - Actual-Gas-Received Population:
| Control (n=116) | NO (n=119) | P value | Absolute rate reduction (%) | Relative rate reduction (%) | |
|---|---|---|---|---|---|
| Death or ECMOa,b | 72 (62%) | 57 (48%) | 0.036 | -14.2 | -22.8 |
| Death | 18 (16%) | 18 (15%) | 1.000 | Not applicable | Not applicable |
| ECMO | 62 (53%) | 48 (40%) | 0.050 | -13.1 | -24.5 |
a Extracorporeal membrane oxygenation
b Death or need for ECMO was the study's primary end point
The response rate (full response, partial response, no response) to 20 ppm inhaled nitric oxide for the actual-gas-received population was also determined in a post-hoc analysis and is presented in Table 3.
Table 3. Response Rate to Study Gas - Actual-Gas-Received Population:
| Response | Placebo (n=112) | NO (n=117) |
|---|---|---|
| Full (>20 torr increase in PaO2 30 minutes) | 16 (14.3%) | 58 (49.6%) |
| Partial (10-20 torr increase in PaO2 30 minutes) | 13 (11.6%) | 17 (14.5%) |
| No (<10 torr increase in PaO2 30 minutes) | 83 (74.1%) | 42 (35.9%) |
Data also showed that only 5.5% of neonates who did not respond, or partially responded to the inhaled nitric oxide therapy at a 20 ppm dose were converted to full response with 80 ppm inhaled nitric oxide, indicating no additional benefit of inhaled nitric oxide at 80 ppm. These findings are consistent with conclusions from the original ITT population.
The rate of death or receipt of ECMO was assessed in a post-hoc analysis of the actual-gasreceived population by initial response to 20 ppm inhaled nitric oxide, and is presented in Table 4.
Table 4. ECMO Receipt by Initial Response Status - Actual-Gas-Received Population:
| Response | Rate of Death or ECMO | ||||
|---|---|---|---|---|---|
| Placebo (n=112) | NO (n=117) | P Value* | Absolute rate reduction (%) | Relative rate reduction (%) | |
| Fully responded in the first 30 minutes** | 8/16 (50%) | 15/58 (25.9%) | 0.076 | -24.1 | -48.2 |
| Partial or no response in the first 30 minutes | 63/96 (65.6%) | 40/59 (67.8%) | 0.862 | 2.2 | 3.4 |
* p-value from Fisher's 2-tailed exact test
** full response was defined as ≥20 mm Hg increase in PaO2 after 30 minutes of gas treatment
These results showed that the rate of death or receipt of ECMO between treatment groups differed according to the initial response to 20 ppm inhaled nitric oxide, indicating that patients who initially did not fully respond to inhaled nitric oxide therapy in the first 30 minutes of treatment did not benefit significantly from the therapy.
The nitric oxide group had significantly greater increases in PaO2 and greater decreases in the OI and the alveolar-arterial oxygen gradient than the control group (p <0.001 for all parameters).
No infant had study drug discontinued for toxicity. Inhaled nitric oxide had no detectable effect on mortality. The adverse events collected in the NINOS trial occurred at similar incidence rates in both treatment groups (See Adverse Reactions).
CINRGI study: This study was a double-blind, randomized, placebo-controlled, multicenter trial of 186 term and late pre-term neonates (≥34 weeks gestational age) with pulmonary hypertension and hypoxic respiratory failure, with OI values of ≥25 cm H2O/mm Hg. The primary objective of the study was to determine whether nitric oxide would reduce the receipt of ECMO in these patients. Hypoxic respiratory failure was caused by MAS (35%), idiopathic PPHN (30%), pneumonia/sepsis (24%), or RDS (8%). Patients with a mean PaO2 of 54 mm Hg and a mean OI of 44 cm H2O / mm Hg were randomly assigned to receive either 20 ppm nitric oxide (n=97) or nitrogen gas (placebo; n=89) in addition to their ventilatory support. Patients who exhibited a PaO2 >60 mm Hg and a pH <7.55 were weaned to 5 ppm nitric oxide or placebo. The maximum duration of nitric oxide therapy was 96 hours. The primary results from the CINRGI study are presented in Table 5.
Table 5. Summary of Clinical Results from CINRGI Study:
| Placebo | NO | P Value* | Absolute rate reduction (%) | Relative rate reduction (%) | |
|---|---|---|---|---|---|
| ECMOa,b | 51/89 (57%) | 30/97 (31%) | <0.001 | -26.4 | -46.0 |
| Death | 5/89 (6%) | 3/97 (3%) | 0.48 | Not applicable | Not applicable |
a Extracorporeal membrane oxygenation
b ECMO was the primary end point of this study
Significantly fewer neonates in the nitric oxide group required ECMO compared to the control group (31% vs. 57%, p <0.001). While the number of deaths were similar in both groups nitric oxide, 3%; placebo, 6%), the combined incidence of death and/or receipt of ECMO was decreased in the nitric oxide group (33% vs. 58%, p <0.001).
In addition, the nitric oxide group had significantly improved oxygenation as measured by PaO2, OI, and alveolar-arterial gradient (p <0.001 for all parameters). Of the 97 patients treated with nitric oxide, 2 (2%) were withdrawn from study drug due to methemoglobin levels >4%. The frequency and number of adverse events reported were similar in the two study groups (See Adverse Reactions).
Detailed pharmacology
Human
Pharmacokinetics
The safety of short-term inhalation of nitric oxide (NO) (40 ppm for 2 hours) in 12 healthy volunteers demonstrated no notable effects on systolic and diastolic blood pressures, heart rate, respiratory rate, or peripheral oxygen saturation. Nor were significant effects on hematologic and chemistry laboratory assessments noted (CTN-NO-93-006). Normal, healthy adult volunteers studies of inhaled nitric oxide at doses of up to 128 ppm, that is greater than any dose used clinically, demonstrate no clinically significant methemoglobinemia. Maximum levels of methemoglobin are achieved after 3 to 5 hours on NO inhalation and pharmacokinetic modeling was performed on the raw data by Ohmeda (RDR 0076). In both healthy subjects and patients with severe heart failure, the metabolism of NO was found to be dependent on the oxygenation of red cell hemoglobin (CTN-NO-93-008). The data indicate that the inactivation of NO occurred in the red blood cells and suggested that oxyhemoglobin acted as an oxygen donor to the NO molecule in its conversion to nitrate. The fraction of NO inactivated vial stoichiometric conversion to nitrate and methemoglobin seemed to be determined by the oxyhemoglobin/hemoglobin ratio in the red blood cells. A study of healthy adult volunteers found that not all of the absorbed NO initially forms methemoglobin, but up to approximately 14% of absorbed NO may be converted directly to nitrogen oxides, which have a volume of distribution equal to about one third of body weight and a clearance similar to the glomerular filtration rate (Young et al2). Data for another study in healthy adult men indicated that the conversion of NO into NO3 - is a major metabolic pathway for inhaled NO in humans and that over 70% of inhaled NO is excreted as NO3 - in the urine (Westfelt et al3).
Pharmacokinetics in Neonates
Methemoglobin formation is expected during treatment with inhaled nitric oxide in the proposed dose range and should be dose-dependent. Patients not receiving inhaled nitric oxide typically have methemoglobin levels of 0.2 to 1%. The primary problem with elevated methemoglobin is that it reduces the total oxygen-carrying capacity of blood. The acceptable levels for methemoglobin are controversial. Most investigators have used 5 to 10% methemoglobin as the maximum acceptable level.
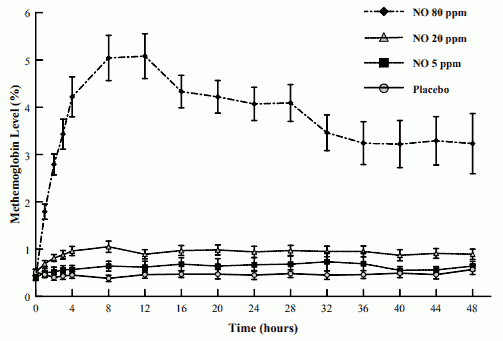
The mean methemoglobin levels for the Ohmeda INO 01/02 trial are shown in Figure 1. As seen, there is a dose-dependent increase in methemoglobin levels with maximal levels of approximately 5% (the predefined level of methemoglobin at which the inhaled nitric oxide dose was to be reduced) in the 80-ppm inhaled nitric oxide dose group. Doses of 20-ppm or less of inhaled nitric oxide, however, had average values for methemoglobin of approximately 1% or less.
FIGURE 1. METHEMOGLOBIN LEVELS - OHMEDA INO 01/02 TRIAL (MEAN ± STANDARD DEVIATION):
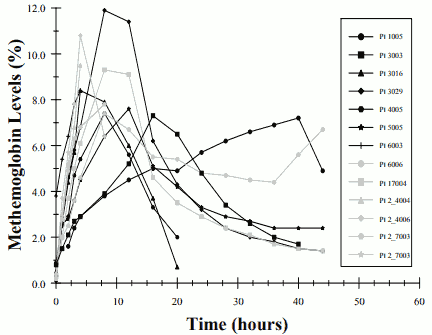
Thirteen of the 37 patients receiving 80-ppm inhaled nitric oxide (35%) in this study developed methemoglobin levels above 7%. The time course for these patients is seen in Figure 2. The mean time to reach their peak level of methemoglobin was 10.5 ± 9.5 hours. Most patients reached this level within the first 18 hours of therapy although one patient did not until 40 hours on inhaled nitric oxide thus emphasizing the need to continue to monitor levels over 48 hours of initiation of therapy. No patient receiving 20-ppm or 5-ppm inhaled nitric oxide in this trial had methemoglobin levels above 7%.
FIGURE 2. METHEMOGLOBIN LEVELS- OHMEDA INO 01/02 TRIAL - PATIENTS WITH METHEMOGLOBINEMIA:
Hemostasis Modifying Agents
Endogenous NO is thought to regulate the platelet cGMP and to have antiaggregatory activity (Radomski et al4). There is also controversy whether the combination of inhaled NO and other pharmaceutical compounds that have anti-coagulative properties may influence hemostasis synergistically or additively. In study ICR 013402 randomized volunteers received either placebo inhalation, or 80 ppm inhaled NO, with or without heparin 5000 E given i.v. at the start of inhalation procedure. In no instance did the combination of inhaled NO + heparin cause a prolonged bleeding time, thus ruling out additive/synergistic effects between inhaled NO and an anti-coagulative agent (heparin).
Pharmacodynamics
In patients who are 'responders' to this therapy in terms of improved arterial oxygen tension during mechanical ventilation, the main pharmacodynamic response to inhaled nitric oxide is typically seen within a few minutes from the start of treatment.
The main effect of inhaled nitric oxide is to relax lung vascular smooth muscle, causing dilation of blood vessels and consequently increased blood flow in the region reached by the compound.
Pharmacodynamics in Neonates:
The improvement of arterial oxygen tension in hypoxemic newborns during administration of inhaled nitric oxide is often due to the combined reduction of both extra-pulmonary and intrapulmonary shunting. The impairment of gas exchange is traditionally estimated by repeated calculations of oxygenation index (OI) in neonates, with OI = 100 x (FiO2 x MAP)/ PaO2, with MAP = mean airway pressure, FiO2 = fraction of inspired oxygen, PaO2 = postductal arterial oxygen tension. Historical control suggests that OI >40 is correlated to 80% mortality and is often used as the threshold value for rescue with ECMO.
Any therapy with a clinically meaningful impact on hypoxemic respiratory failure should thus cause a significant reduction of OI, preferably a sustained reduction below 40, which would indicate establishment of acceptable oxygenation requiring less aggressive ventilator settings. The sponsor conducted a dose finding study in neonatal patients (CTN-NO-93-003), which demonstrated a rapid (within 10 minutes) improvement in arterial oxygenation already at dose at or below 10 ppm in a majority of neonates.
Animal
From a study in dogs it can be deduced that the lethal concentration is around 640 ppm nitric oxide for 4 hours, whereas, exposures of 320 ppm nitric oxide are non-lethal (Study SC940065).
Toxicology
The preclinical safety profile of nitric oxide was assessed in rats in repeat dose inhalation studies up to 2 years in duration. Age-specific nitric oxide-induced toxicity has not been determined, as juvenile animal toxicity studies were not conducted. There are no reproductive animal studies or human studies to evaluate nitric oxide for effects on fertility or harm to the developing fetus. Nitric oxide has demonstrated genotoxicity in some bacterial strains used in the Salmonella (Ames Test), the mouse lymphoma test, Chinese hamster ovary cell test, in vivo exposure in rats, and human lymphocytes.
Inhalation exposures of F344 rats to 20, 10 or 5 ppm NO for 20 hr/day for up two years were examined. The results of this study indicate that there was no clear evidence of a toxic effect on the respiratory tract or other organs as determined using clinical and ophthalmoscopic observations, examination of tissues at necropsy, organ and body weight changes, clinical pathology, and histopathologic examination of tissues.
Repeat Dose (Long-Term) Toxicology:
| Reports | Species & Test System | Dose/Concentration | Study Type & Duration | Comments |
|---|---|---|---|---|
| SC940063 Seven-day range-finding study of Nitric Oxide (NO) in the rat via inhalation. | Sprague-Dawley rats | 0, 80, 200, 300, 400, 500 ppm NO in air | Nose-only inhalation exposures for 6 hrs/day for up to 7 days | No adverse effects below 200 ppm; dose-related increases in metheme above 200 ppm. Histotoxic anoxia due to metheme leading to lethality above 200 ppm. |
| RDR-0149 DS Seven-day range-finding study of Nitric Oxide (NO) in the rat via inhalation Supplement Report | Sprague-Dawley rats | 0, 200, NO in air, with 2.2 ppm NO2 in 200 ppm NO group | Report of evaluation of respiratory tract at the level of electronmicroscopy from animals exposed for 1 or 7 days | Moderate increase of interstitial edema after 1 day, Slight increase after 7 days. Findings consistent with NO2 exposure |
| SC940064 Twenty-eight day exposure with recovery of nitric oxide (NO) in the rat via inhalation. | Sprague-Dawley rats | 0, 40, 80, 160, 200, 250 ppm NO in air with up to 3.5 ppm NO2 in 250 ppm NO group | Nose-only inhalation exposures for 6 hrs/day for 28 days, with 28 day recovery groups | Exposure-system related elevated dosing excursion (32% on day 14-15); lethality at 200 ppm (n=1) and 250 (n=17); dose-related increase in metheme from 160 ppm; metheme levels consistent at 7, 14, 21, 28 days; no systemic histopathologic nor hematologic changes |
| RDR-0150-DS Twenty-eight day exposure with recovery of nitric oxide (NO) in the rat via inhalation. Supplement report | Sprague-Dawley rats | 0, 200, NO in air, with 2.6 ppm NO2 in 200 ppm NO group | Report of evaluation of respiratory tract at the level of electronmicroscopy from animals exposed for 28 days | Slight ultrastructural changes of ciliated respiratory, type 2 alveolar, and clara cells consistent with NO2 exposure. |
Mutagenicity:
| Reports | Category and Test System | Dose/Concentration | Study Type & Duration | Comments |
|---|---|---|---|---|
| 1303/001-1052 Nitric Oxide: Reverse mutation in histidinerequiring strains of Salmonella typhimurium and tryptophan-requiring strains of Escherichia coli. | In vitro/Salmonella typhimurium (TA 98, TA 100, TA 1535, TA 1537) and E. coli (WP2plcM 101, WP2uvrApKM101); with and without S-9 activation | Up to 5,000 ppm NO under continuous flow; ~1 ppm NO2 | Reverse mutation in bacteria | No toxicity |
| 1303/007-1052 Nitrogen dioxide: Reverse mutation in two histidine-requiring strains of Salmonella typhimurium. | In vitro/Salmonella typhimurium (TA 100, TA 1535) with and w/out S-9 activation | Up to 40 ppm NO2 | Reverse mutation in bacteria | Mutagenic with and without S-9 activation from 10 ppm NO2 |
| 1303/002-1052 Nitric oxide: Mutation at the thymidine kinase (tk) locus of mouse lymphoma L5178Y cells using the microtitre- fluctuation technique. | In vitro mammalian cell culture (mouse lymphoma-L5178Y cells) using a liquid medium exposure | Up to 2450 ppm NO in nitrogen | Mutation of thymidine kinase locus in cultured mouse cells | Mutagenic above 125 ppm |
| 1303/5-1052 Nitric oxide: Induction of chromosome aberrations in cultured chinese hamster ovary (CHO) cells. | In vitro chromosome aberration in cultured chinese hamster ovary cells (CHO) | Flow thru system with up to 1800 ppm NO in nitrogen | Mitotic inhibition and chromosomal aberration | 1650 ppm NO yielded mitotic inhibition of 52% and increase in structural damage to chromosomes. |
| 1303/4-1052 Nitric oxide: Induction of chromosome aberrations in the peripheral blood lymphocytes of human volunteers after exposure in vivo. | In vivo human exposures | 40 ppm NO in 30% O2 for 2 hrs | Metaphase analysis | No evidence of chromosomal damage |
| Nguyen et al, 1992. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc Natl Acad Sci USA 1992;89:3030-3034. | TK6 human lymphoblasts | 0.125, 0.25, 0.375 ml NO gas/ml culture medium for 1 h | Mutation at HPRT and TK locus | Positive mutagenesis and single-strand DNA breaks |
Chronic Toxicity and Carcinogenicity Study:
| Reports | Species & Test System | Dose/Concentration | Study Type & Duration | Comments |
|---|---|---|---|---|
| N005243 Chronic Toxicity and Carcinogenicity Study of Nitric Oxide in Male and Female Rats | F344 Rats | 0, 5, 20, and 20 ppm NO in air | Whole-body inhalation exposures for 20 hr/day for up to 2 years | Not carcinogenic |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.