KYLEENA Intrauterine delivery system (IUS) Ref.[7744] Active ingredients: Levonorgestrel
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2019 Publisher: Bayer plc, 400 South Oak Way, Reading, RG2 6AD
Therapeutic indications
Contraception for up to 5 years.
Posology and method of administration
Posology
Kyleena is inserted into the uterine cavity and is effective for up to 5 years.
Insertion and removal/replacement
It is recommended that Kyleena should only be inserted by physicians/healthcare professionals who are experienced in IUS insertions and/or have undergone training on the Kyleena insertion procedure.
Kyleena is to be inserted into the uterine cavity within 7 days of the onset of menstruation. Kyleena can be replaced by a new system at any time in the cycle. Kyleena can also be inserted immediately after first trimester abortion.
Postpartum insertions should be postponed until the uterus is fully involuted, however not earlier than 6 weeks after delivery. If involution is substantially delayed, consider waiting until 12 weeks postpartum.
In case of a difficult insertion and/ or exceptional pain or bleeding during or after insertion, the possibility of perforation should be considered and appropriate steps should be taken, such as physical examination and ultrasound. Physical examination may not be sufficient to exclude partial perforation.
Kyleena can be distinguished from other IUSs by the combination of the visibility of the silver ring on ultrasound and the blue colour of the removal threads. The T-frame of Kyleena contains barium sulfate which makes it visible in X-ray examination.
Kyleena is removed by gently pulling on the threads with a forceps. If the threads are not visible and the system is found to be in the uterine cavity on ultrasound examination, it may be removed using a narrow forceps. This may require dilatation of the cervical canal or surgical intervention.
The system should be removed no later than by the end of the fifth year. If the woman wishes to continue using the same method, a new system can be inserted immediately following removal of the original system.
If pregnancy is not desired, the removal should be carried out within 7 days of the onset of menstruation, provided the woman is experiencing regular menses. If the system is removed at some other time during the cycle or the woman does not experience regular menses and the woman has had intercourse within a week, she is at risk of pregnancy. To ensure continuous contraception a new system should be immediately inserted or an alternative contraceptive method should have been initiated.
After removal of Kyleena, the system should be examined to ensure that it is intact.
Elderly
Kyleena is not indicated for use in postmenopausal women.
Hepatic impairment
Kyleena has not been studied in women with hepatic impairment. Kyleena is contraindicated in women with acute liver disease or liver tumour (see section 4.3).
Renal impairment
Kyleena has not been studied in women with renal impairment.
Paediatric population
Use of this product before menarche is not indicated. For data on safety and efficacy in adolescents, see section 5.1.
Method of administration
To be inserted by a healthcare professional using aseptic technique.
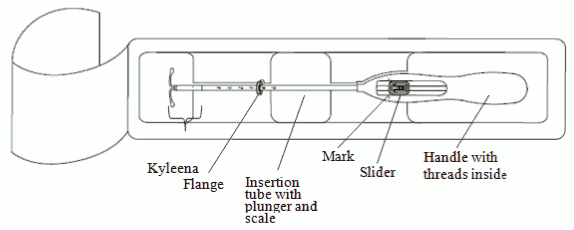
Kyleena is supplied in a sterile package within an integrated inserter that enables single handed loading. The package should not be opened until needed for insertion. Do not resterilize. As supplied, Kyleena is for single use only. Do not use if the blister is damaged or open. Do not insert after the expiry date which is stated on the carton and the blister after EXP.
Any unused product or waste material should be disposed of in accordance with local requirements.
Kyleena is supplied with a patient reminder card in the outer carton. Complete the patient reminder card and give it to the patient, after insertion.
Preparation for insertion
- Examine the patient to establish the size and position of the uterus, in order to detect any signs of acute genital infections or other contraindications for the insertion of Kyleena. If there is any doubt regarding pregnancy, a pregnancy test should be performed.
- Insert a speculum, visualize the cervix, and then thoroughly cleanse the cervix and vagina with a suitable antiseptic solution.
- Employ an assistant as necessary.
- Grasp the anterior lip of the cervix with a tenaculum or other forceps to stabilize the uterus. If the uterus is retroverted, it may be more appropriate to grasp the posterior lip of the cervix. Gentle traction on the forceps can be applied to straighten the cervical canal. The forceps should remain in position and gentle counter traction on the cervix should be maintained throughout the insertion procedure.
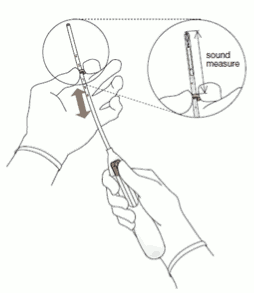
- Advance a uterine sound through the cervical canal to the fundus to measure the depth and confirm the direction of the uterine cavity and to exclude any evidence of intrauterine abnormalities (e.g. septum, submucous fibroids) or a previously inserted intrauterine contraceptive which has not been removed. If difficulty is encountered, consider dilatation of the canal. If cervical dilatation is required, consider using analgesics and/or a paracervical block.
Insertion
1. First, open the sterile package completely (Figure 1). Then use aseptic technique and sterile gloves.
Figure 1:
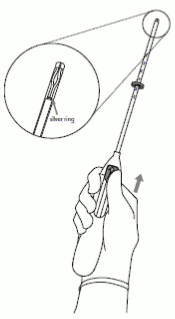
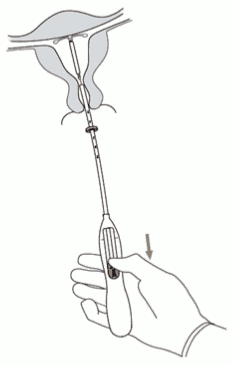
2. Push the slider forward in the direction of the arrow to the furthest position to load Kyleena into the insertion tube (Figure 2).
Figure 2:
IMPORTANT! Do not pull the slider downwards as this may prematurely release Kyleena. Once released, Kyleena cannot be re-loaded.
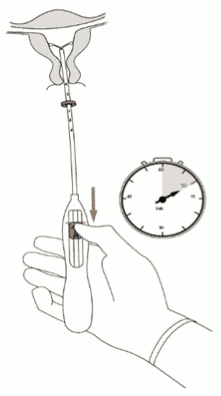
3. Holding the slider in the furthest position, set the upper edge of the flange to correspond to the sound measurement of the uterine depth (Figure 3).
Figure 3:
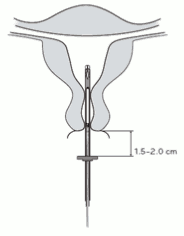
4. While holding the slider in the furthest position, advance the inserter through the cervix until the flange is approximately 1.5-2.0 cm from the uterine cervix (Figure 4).
Figure 4:
IMPORTANT! Do not force the inserter. Dilate the cervical canal, if necessary.
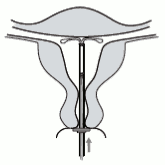
5. While holding the inserter steady, pull the slider to the mark to open the horizontal arms of Kyleena (Figure 5). Wait 5-10 seconds for the horizontal arms to open completely.
Figure 5:
6. Advance the inserter gently towards the fundus of the uterus until the flange touches the cervix. Kyleena is now in the fundal position (Figure 6).
Figure 6:
7. Holding the inserter in place, release Kyleena by pulling the slider all the way down (Figure 7). While holding the slider all the way down, gently remove the inserter by pulling it out. Cut the threads to leave about 2-3 cm visible outside of the cervix.
Figure 7:
IMPORTANT! Should you suspect that the system is not in the correct position, check placement (e.g. with ultrasound). Remove the system if it is not positioned properly within the uterine cavity. A removed system must not be re-inserted.
Removal/replacement
For removal/replacement, please see section 4.2 Insertion and removal/replacement.
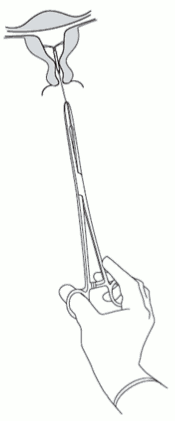
Kyleena is removed by pulling on the threads with a forceps (Figure 8).
You may insert a new Kyleena immediately following removal.
After removal of Kyleena, the system should be examined to ensure that it is intact.
Figure 8:
Overdose
Not relevant.
Shelf life
Shelf life: 3 years.
Special precautions for storage
This medicinal product does not require any special storage conditions.
Nature and contents of container
The product is individually packed into a thermoformed blister package (PETG) with a peelable lid (PE).
Pack sizes: 1x1 and 5x1.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
The product is supplied in a sterile package which should not be opened until required for insertion. Each system should be handled with aseptic precautions. If the seal of the sterile envelope is broken, the system inside should be disposed of in accordance with local guidelines for the handling of biohazardous waste. Likewise, a removed Kyleena and inserter should be disposed of in this manner.
To be inserted by a healthcare professional using aseptic technique (see section 4.2).
Any unused medicinal product or waste material should be disposed of in accordance with local requirements. This medicinal product may pose a risk to the environment (see section 5.3).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.