LATISSE Eyes drops, solution Ref.[10860] Active ingredients: Bimatoprost
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
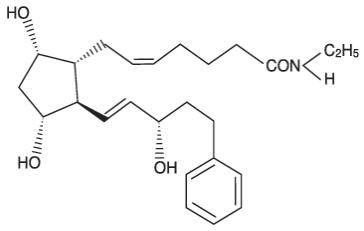
LATISSE (bimatoprost ophthalmic solution) 0.03% is a synthetic prostaglandin analog. Its chemical name is (Z)7[(1R,2R,3R,5S)-3,5-Dihydroxy-2-[(1E,3S)-3-hydroxy-5-phenyl-1-pentenyl]cyclopentyl]-N-ethyl-5-heptenamide, and its molecular weight is 415.58. Its molecular formula is C25H37NO4.
Its chemical structure is:
Bimatoprost is a powder, which is very soluble in ethyl alcohol and methyl alcohol and slightly soluble in water. LATISSE is a clear, isotonic, colorless, sterile ophthalmic solution with an osmolality of approximately 290 mOsmol/kg.
Contains:
Active: bimatoprost 0.3 mg/mL
Preservative: benzalkonium chloride 0.05 mg/mL
Inactives: sodium chloride; sodium phosphate, dibasic; citric acid; and purified water.
Sodium hydroxide and/or hydrochloric acid may be added to adjust pH. The pH during its shelf life ranges from 6.8-7.8.
| Dosage Forms and Strengths |
|---|
|
Ophthalmic solution containing bimatoprost 0.3 mg/mL. |
| How Supplied |
|---|
|
LATISSE (bimatoprost ophthalmic solution) 0.03% is supplied sterile in opaque white low density polyethylene dispenser bottles and tips with turquoise polystyrene caps accompanied by sterile, disposable applicators: 3 mL in a 5 mL bottle with 70 applicators NDC 0023-3616-70 Distributed by: Allergan USA, Inc., Madison, NJ 07940 |
Drugs
| Drug | Countries | |
|---|---|---|
| LATISSE | Brazil, Canada, New Zealand, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.