LEVAQUIN Film-coated tablet Ref.[49676] Active ingredients: Levofloxacin
Source: FDA, National Drug Code (US) Revision Year: 2011
Product description
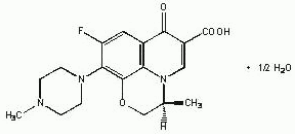
LEVAQUIN is a synthetic broad-spectrum antibacterial agent for oral and intravenous administration. Chemically, levofloxacin, a chiral fluorinated carboxyquinolone, is the pure (-) - (S) - enantiomer of the racemic drug substance ofloxacin. The chemical name is (-) - (S) -9-fluoro-2,3-dihydro-3-methyl-10-(4- methyl-1-piperazinyl)-7-oxo-7H-pyrido[1,2,3-de]-1,4-benzoxazine-6-carboxylic acid hemihydrate.
Figure 1. The Chemical Structure of Levofloxacin:
The empirical formula is C18H20FN3O4•½H2O and the molecular weight is 370.38. Levofloxacin is a light yellowish-white to yellow-white crystal or crystalline powder. The molecule exists as a zwitterion at the pH conditions in the small intestine.
The data demonstrate that from pH 0.6 to 5.8, the solubility of levofloxacin is essentially constant (approximately 100 mg/mL). Levofloxacin is considered soluble to freely soluble in this pH range, as defined by USP nomenclature. Above pH 5.8, the solubility increases rapidly to its maximum at pH 6.7 (272 mg/mL) and is considered freely soluble in this range. Above pH 6.7, the solubility decreases and reaches a minimum value (about 50 mg/mL) at a pH of approximately 6.9.
Levofloxacin has the potential to form stable coordination compounds with many metal ions. This in vitro chelation potential has the following formation order: Al+3 >Cu+2 >Zn+2 >Mg+2 >Ca+2.
Excipients and Des cription of Dosage Forms
LEVAQUIN Tablets
LEVAQUIN Tablets are available as film-coated tablets and contain the following inactive ingredients:
- 250 mg (as expressed in the anhydrous form): hypromellose, crospovidone, microcrystalline cellulose, magnesium stearate, polyethylene glycol, titanium dioxide, polysorbate 80 and synthetic red iron oxide.
- 500 mg (as expressed in the anhydrous form): hypromellose, crospovidone, microcrystalline cellulose, magnesium stearate, polyethylene glycol, titanium dioxide, polysorbate 80 and synthetic red and yellow iron oxides.
- 750 mg (as expressed in the anhydrous form): hypromellose, crospovidone, microcrystalline cellulose, magnesium stearate, polyethylene glycol, titanium dioxide, polysorbate 80.
LEVAQUIN Oral Solution
LEVAQUIN Oral Solution, 25 mg/mL, is a multi-use self-preserving aqueous solution of levofloxacin with pH ranging from 5.0 to 6.0. The appearance of LEVAQUIN Oral Solution may range from clear yellow to clear greenish-yellow. This does not adversely affect product potency.
LEVAQUIN Oral Solution contains the following inactive ingredients: sucrose, glycerin, sucralose, hydrochloric acid, purified water, propylene glycol, artificial and natural flavors, benzyl alcohol, ascorbic acid, and caramel color. It may also contain a solution of sodium hydroxide for pH adjustment.
LEVAQUIN Injection
The appearance of LEVAQUIN Injection may range from a clear yellow to a clear greenish-yellow solution. This does not adversely affect product potency.
LEVAQUIN Injection in Single-Use Vials is a sterile, preservative-free aqueous solution of levofloxacin in Water for Injection, with pH ranging from 3.8 to 5.8.
LEVAQUIN Injection Premix in Single-Use Flexible Containers is a sterile, preservative-free aqueous solution of levofloxacin with pH ranging from 3.8 to 5.8. This is a dilute, non-pyrogenic, nearly isotonic premixed solution that contains levofloxacin in 5% Dextrose (D~5~W). Solutions of hydrochloric acid and sodium hydroxide may have been added to adjust the pH.
The flexible container is fabricated from a specially formulated non-plasticized, thermoplastic copolyester (CR3). The amount of water that can permeate from the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the flexible container can leach out certain of the container’s chemical components in very small amounts within the expiration period. The suitability of the container material has been confirmed by tests in animals according to USP biological tests for plastic containers.
| Dosage Forms and Strengths |
|---|
|
TABLETS, Film-coated, capsule-shaped:
ORAL SOLUTION, 25mg/mL, clear yellow to clear greenish-yellow color INJECTION, Single-Use Vials of concentrated solution for dilution for intravenous infusion, clear yellow to clear greenish-yellow in appearance:
INJECTION (5 mg/mL in 5% Dextrose) Premix in Single-Use Flexible Containers, for intravenous infusion:
|
| How Supplied | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
16.1 LEVAQUIN TabletsLEVAQUIN Tablets are supplied as 250, 500, and 750 mg capsule-shaped, coated tablets. LEVAQUIN Tablets are packaged in bottles and in unit-dose blister strips in the following configurations: 250 mg tablets are terra cotta pink and are imprinted: “LEVAQUIN” on one side and “250” on the other side.
LEVAQUIN Tablets are manufactured for PriCara, Division of Ortho-McNeil-Janssen Pharmaceuticals, Inc., Raritan, NJ 08869 by Janssen Ortho LLC, Gurabo, Puerto Rico 00778. Relabeling and Repackaging by: Physicians Total Care, Inc., Tulsa, OK 74146 |
Drugs
| Drug | Countries | |
|---|---|---|
| LEVAQUIN | Brazil, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.