LEXAPRO Tablet / Oral solution Ref.[11115] Active ingredients: Escitalopram
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
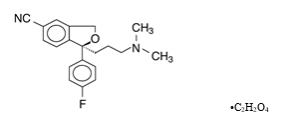
Lexapro contains escitalopram, an orally administered selective serotonin reuptake inhibitor (SSRI). Escitalopram is the pure S-enantiomer (single isomer) of the racemic bicyclic phthalane derivative citalopram. Escitalopram oxalate is designated S-(+)1[3(dimethyl-amino)propyl]1(p-fluorophenyl)-5-phthalancarbonitrile oxalate with the following structural formula:
The molecular formula is C20H21FN2O • C2H2O4 and the molecular weight is 414.40.
Escitalopram oxalate occurs as a fine, white to slightly-yellow powder and is freely soluble in methanol and dimethyl sulfoxide (DMSO), soluble in isotonic saline solution, sparingly soluble in water and ethanol, slightly soluble in ethyl acetate, and insoluble in heptane.
Lexapro is available as tablets for oral administration.
Lexapro tablets are film-coated, round tablets containing 6.38 mg, 12.75 mg, 25.50 mg escitalopram oxalate in strengths equivalent to 5 mg, 10 mg, and 20 mg, respectively, of escitalopram base. The 10 and 20 mg tablets are scored. The tablets also contain the following inactive ingredients: talc, croscarmellose sodium, microcrystalline cellulose/colloidal silicon dioxide, and magnesium stearate. The film coating contains hypromellose, titanium dioxide, and polyethylene glycol.
Lexapro oral solution contains 1.29 mg/ml escitalopram oxalate equivalent to 1 mg/mL escitalopram base. It also contains the following inactive ingredients: sorbitol, purified water, citric acid, sodium citrate, malic acid, glycerin, propylene glycol, methylparaben, propylparaben, and natural peppermint flavor. The oral solution is not currently marketed.
| Dosage Forms and Strengths |
|---|
TabletsLexapro tablets are film-coated, round tablets containing escitalopram in strengths equivalent to 5 mg, 10 mg and 20 mg escitalopram base. The 10 and 20 mg tablets are scored. Imprinted with “FL” on one side and either “5”, “10”, or “20” on the other side according to their respective strengths. Oral SolutionLexapro oral solution contains escitalopram equivalent to 1 mg/mL escitalopram base (not currently being marketed). |
| How Supplied |
|---|
Tablets5 mg Tablets: Bottle of 100 NDC # 0456-2005-01 White to off-white, round, non-scored, film-coated. Imprint “FL” on one side of the tablet and “5” on the other side. 10 mg Tablets: Bottle of 100 NDC # 0456-2010-01 10 × 10 Unit Dose NDC # 0456-2010-63 White to off-white, round, scored, film-coated. Imprint on scored side with “F” on the left side and “L” on the right side. Imprint on the non-scored side with “10”. 20 mg Tablets: Bottle of 100 NDC # 0456-2020-01 10 × 10 Unit Dose NDC # 0456-2020-63 White to off-white, round, scored, film-coated. Imprint on scored side with “F” on the left side and “L” on the right side. Imprint on the non-scored side with “20”. Oral Solution5 mg/5 mL, peppermint flavor (240 mL) NDC # 0456-2101-08. The oral solution is not currently being marketed. Distributed by: Allergan USA, Inc., Madison, NJ 07940 |
Drugs
| Drug | Countries | |
|---|---|---|
| LEXAPRO | Australia, Brazil, Ecuador, Hong Kong, Ireland, Japan, Mexico, Netherlands, New Zealand, Poland, Singapore, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.