LIPANTHYL Penta Film-coated tablet Ref.[50342] Active ingredients: Fenofibrate
Source: Health Sciences Authority (SG) Revision Year: 2022 Publisher: Abbott Products Operations AG, Hegenheimermattweg 127, 4123 Allschwil, Switzerland
5.1. Pharmacodynamic properties
Serum Lipid Reducing Agents/Cholesterol and Triglycerides Reducers/Fibrates
ATC code: C10AB05
Dyslipidaemia
Fenofibrate is a fibric acid derivative whose lipid modifying effects reported in humans are mediated via activation of Peroxisome Proliferator Activated Receptor type alpha (PPARα).
Through activation of PPARα, fenofibrate increases the lipolysis and elimination of atherogenic triglyceride-rich particles from plasma by activating lipoprotein lipase and reducing production of apoprotein CIII. Activation of PPARα also induces an increase in the synthesis of apoproteins AI and AII.
The above stated effects of fenofibrate on lipoproteins lead to a reduction in very low- and low density fractions (VLDL and LDL) containing apoprotein B and an increase in the high density lipoprotein fraction (HDL) containing apoprotein AI and AII.
In addition, through modulation of the synthesis and the catabolism of VLDL fractions fenofibrate increases the LDL clearance and reduces small dense LDL, the levels of which are elevated in the atherogenic lipoprotein phenotype, a common disorder in patients at risk for coronary heart disease.
During clinical trials with fenofibrate, total cholesterol was reduced by 20 to 25%, triglycerides by 40 to 55% and HDL cholesterol was increased by 10 to 30%.
In hypercholesterolaemic patients, where LDL cholesterol levels are reduced by 20 to 35%, the overall effect on cholesterol results in a decrease in the ratios of total cholesterol to HDL cholesterol, LDL cholesterol to HDL cholesterol, or Apo B to Apo AI, or a decrease of the levels of non-HDL cholesterol all of which are markers of atherogenic risk.
Extravascular deposits of cholesterol (tendinous and tuberous xanthoma) may be markedly reduced or even entirely eliminated during fenofibrate therapy. Patients with raised levels of fibrinogen treated with fenofibrate have shown significant reductions in this parameter, as have those with raised levels of Lp(a). Other inflammatory markers such as C Reactive Protein are reduced with fenofibrate treatment.
The uricosuric effect of fenofibrate leading to reduction in uric acid levels of approximately 25% should be of additional benefit in those dyslipidaemic patients with hyperuricaemia.
Fenofibrate has been shown to possess an anti-aggregatory effect on platelets in animals and in a clinical study, which showed a reduction in platelet aggregation induced by ADP, arachidonic acid and epinephrine.
Diabetic Retinopathy
Several mechanisms have been proposed to explain the effects of fenofibrate in proliferative diabetic retinopathy (PDR) and diabetic macular edema (DME) in vitro and in rodent models. Fenofibrate was shown to reduce the retinal expression of VEGF, the major angiogenic factor in PDR and to reduce vascular permeability and apoptosis of retinal pigmented epithelium, which contributes to development of DME.
The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study was a multinational randomized trial of 9795 patients with type 2 diabetes mellitus. Eligible patients were randomly assigned to receive fenofibrate 200 mg/day (n=4895) or matching placebo (n=4900). In a sub study of 1012 patients (ophthalmology sub study), standardized retinal photography was done and photographs graded with Early Treatment Diabetic Retinopathy Study (ETDRS) criteria to determine the cumulative incidence of diabetic retinopathy and its component lesions. Analyses were by intention to treat. In the ophthalmology sub study, the primary endpoint of 2-step progression of retinopathy grade did not differ significantly between the two groups overall (46 [9·6%] patients on fenofibrate vs 57 [12·3%] on placebo; p=0·19) or in the subset of patients without pre-existing retinopathy (43 [11·4%] vs 43 [11·7%]; p=0·87). By contrast, in patients with pre-existing retinopathy, significantly fewer patients on fenofibrate had a 2-step progression than did those on placebo (three [3·1%] patients vs 14 [14·6%]; p=0·004).
At each clinic visit, information concerning laser treatment for diabetic retinopathy-a prespecified tertiary endpoint of the main study-was gathered. The requirement for first laser treatment for all retinopathy was significantly lower in the fenofibrate group than in the placebo group (164 [3·4%] patients on fenofibrate vs. 238 [4·9%] on placebo; hazard ratio [HR] 0·69, 95% CI 0·56–0·84; p=0·0002; absolute risk reduction 1·5% [0·7-2·3]). The need for such treatment was not affected by plasma lipid concentrations.
In a subgroup of 2856 participants of the ACCORD study, ACCORD Eye evaluated the effects of three interventions strategies, on the progression of diabetic retinopathy; intensive or standard treatment for glycaemia (target HbA1c <6.0% or 7.0 to 7.9%, respectively), dyslipidaemia (160 mg daily of fenofibrate plus simvastatin or placebo plus simvastatin) or systolic blood-pressure control (target, <120 or <140 mm Hg). Progression of diabetic retinopathy was defined at 4 years by 3 or more steps on the ETDRS scale (as assessed from seven-field stereoscopic fundus photographs) or the development of diabetic retinopathy necessitating laser photocoagulation or vitrectomy.
The rate of progression of diabetic retinopathy was 6.5% with fenofibrate for intensive dyslipidaemia therapy, versus 10.2% with placebo (adjusted odds ratio, 0.60; 95% CI, 0.42 to 0.87; P = 0.006). It was concluded that intensive combination treatment of dyslipidaemia reduced the rate of progression of diabetic retinopathy.
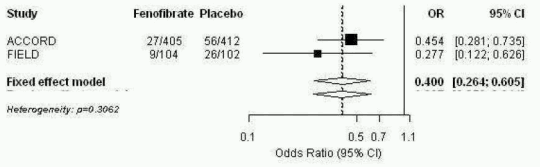
An integrated analysis was performed from Patient individual Data from the FIELD study and the published information from ACCORD Eye publications. The combined primary endpoint of ACCORD Eye was applied to FIELD i.e.3-step ETDRS severity scale, photocoagulation or vitrectomy for proliferative diabetic retinopathy. The two studies were homogeneous (fixedeffect model applicable) and showed an overall 60% reduction in the progression of diabetic retinopathy, OR: 0.40; 95% CI (0.26-0.61) for subjects with existing DR at baseline.
Figure 1. Progression of Diabetic Retinopathy (DR) in Subjects with DR at Baseline – Combined Analysis of FIELD PSP-DR and ACCORD Eye for ACCORD Eye Primary Endpoint:
FIELD and ACCORD studies excluded patients with severe non-proliferative and proliferative diabetic retinopathy at baseline.
5.2. Pharmacokinetic properties
Metabolism and excretion
LIPANTHYL PENTA 145, film-coated tablet contains 145 mg of fenofibrate nanoparticles.
Absorption
Maximum plasma concentrations (Cmax) occur within 2 to 4 hours after oral administration. Plasma concentrations are stable during continuous treatment in any given individual.
Contrarily to previous fenofibrate formulations, the maximum plasma concentration and overall exposure of the nanoparticle formulation is independent from food intake. Therefore, LIPANTHYL PENTA 145, film-coated tablet may be taken without regard to meals.
A food-effect study involving administration of the new 145 mg tablet formulation of fenofibrate to healthy male and female subjects under fasting conditions and with a high fat meal indicated that exposure (AUC and Cmax) to fenofibric acid is not affected by food.
Distribution
Fenofibric acid is strongly bound to plasma albumin (more than 99%).
Metabolism and excretion
After oral administration, fenofibrate is rapidly hydrolysed by esterases to the active metabolite fenofibric acid. No unchanged fenofibrate can be detected in the plasma. Fenofibrate is not a substrate for CYP3A4. No hepatic microsomal metabolism is involved.
The drug is excreted mainly in the urine. Practically all the drug is eliminated within 6 days. Fenofibrate is mainly excreted in the form of fenofibric acid and its glucuronide conjugate. In elderly patients, the fenofibric acid apparent total plasma clearance is not modified.
Kinetic studies following the administration of a single dose and continuous treatment have demonstrated that the drug does not accumulate. Fenofibric acid is not eliminated by haemodialysis.
The plasma elimination half-life of fenofibric acid is approximately 20 hours.
5.3. Preclinical safety data
Acute toxicity studies have yielded no relevant information about specific toxicity of fenofibrate.
In a three-month oral nonclinical study in the rat species with fenofibric acid, the active metabolite of fenofibrate, toxicity for the skeletal muscles (particularly those rich in type I – slow oxidativemyofibres) and cardiac degeneration, anemia and decreased body weight were seen at exposure levels ≥50- fold the human exposure for the skeletal toxicity and >15 fold for the cardiomyotoxicity.
Reversible ulcers and erosions in the gastro-intestinal tract occurred in dogs treated during 3 months at exposures approximately 7-fold the clinical AUC.
Studies on mutagenicity have been negative.
In rats and mice, liver tumours have been found at high dosages, which are attributable to peroxisome proliferation. These changes are specific to small rodents and have not been observed in other animal species. This is of no relevance to therapeutic use inman.
Studies in mice, rats and rabbits did not reveal any teratogenic effect. Embryotoxic effects were observed at doses in the range of maternal toxicity. Prolongation of the gestation period and difficulties during delivery were observed at high doses.
No effects on fertility were detected in non-clinical reproductive toxicity studies conducted with fenofibrate. However reversible hypospermia and testicular vacuolation and immaturity of the ovaries were observed in a repeat-dose toxicity study with fenofibric acid in young dogs.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.