LOARGYS Solution for injection/infusion Ref.[107905] Active ingredients: Pegzilarginase
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: Immedica Pharma AB, 113 63 Stockholm, Sweden
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other alimentary tract and metabolism products, enzymes
ATC code: A16AB24
Mechanism of action
ARG1-D is an inherited metabolic disease characterised by deficiency of the arginase 1 enzyme and associated with the persistent elevation of plasma arginine leading to disease manifestations and progression of clinical symptoms.
Pegzilarginase is a cobalt substituted recombinant human arginase 1 enzyme conjugated with 5 kDa mPEG carriers at a degree of substitution of 6-12 moles of mPEG per mole of protein. The molecular mass of the conjugated protein is approximately 224-344 kdA. The mPEG carrier reduces clearance of pegzilarginase resulting in an extended half-life while maintaining the functions of the enzyme. Pegzilarginase is intended to substitute for the deficient human arginase 1 enzyme activity in patients with ARG1-D. Pegzilarginase has been shown to rapidly and sustainably reduce plasma arginine and convert it to urea and ornithine.
Pharmacodynamic effects
The PD effects of pegzilarginase have been evaluated in adults and paediatric subjects with ARG1-D across a range of doses administered both intravenously and subcutaneously.
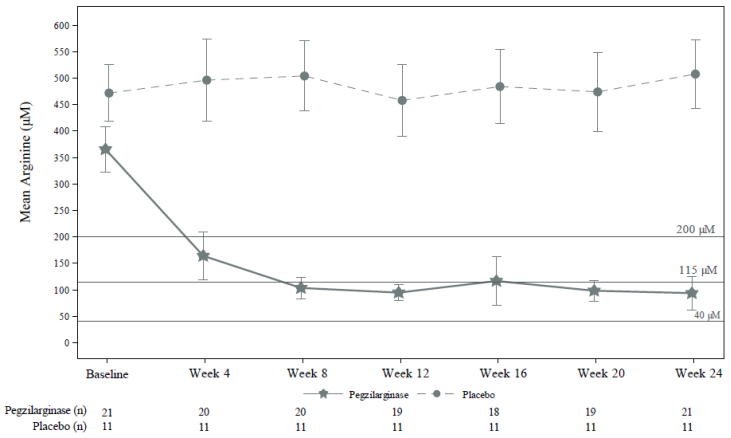
Intravenous administration of pegzilarginase resulted in early reductions in plasma arginine levels with median time to nadir (lowest arginine level) of 2-5 hours. It is expected that plasma arginine will reach its steady-state on or before Week 8 (see Figure 1). It is not expected for the time to reach these levels to be influenced by the baseline plasma arginine value or the route of administration.
Plasma arginine levels remained controlled after switching from intravenous to subcutaneous administration at the same dose, and subcutaneous administration led to fewer and shorter episodes of pegzilarginase-induced hypoargininaemia.
Corresponding significant increases in plasma ornithine levels and decreases in plasma guanidino compound levels were demonstrated with pegzilarginase treatment. Treatment with pegzilarginase does not directly target elevated plasma ammonia levels.
Clinical efficacy and safety
The safety and efficacy of pegzilarginase were assessed in a multicentre, double-blind, placebocontrolled trial (CAEB1102-300A, ‘Study 300A’) which included 32 paediatric and adult subjects aged 2 to 29 years with ARG1-D. Subjects were randomised 2:1 to receive pegzilarginase or placebo intravenously once weekly at an initial dose of 0.1 mg/kg and titrated within a range of 0.05 mg to 0.2 mg/kg. All subjects were to continue on any previously prescribed dietary regimen and ammonia scavengers throughout the trial period.
The primary endpoint assessed the reduction from baseline in plasma arginine in subjects treated with pegzilarginase compared to placebo at Week 24. The key secondary endpoints assessing functional mobility were Gross Motor Function Measure Part E (GMFM-E, walking, running, jumping) and the 2-minute walk test (2MWT). Additionally, the proportion of subjects achieving plasma arginine levels below target per treatment guidelines (<200 µM) and within the normal range as well as the effect on GMFM Part D (GMFM-D, standing) were evaluated as secondary endpoints.
Treatment with pegzilarginase resulted in a statistically significant reduction in plasma arginine compared to placebo (p<0.0001) after 24 weeks of treatment (Table 2 and Figure 1). Plasma arginine levels below guideline recommended target and within normal range were achieved in 90.5% of pegzilarginase-treated subjects compared to 0% of the subjects in the placebo arm (Table 2 and Figure 1).
Table 2. Analysis of plasma arginine endpoints during Study 300A double-blind period:
| Pegzilarginase (n=21) | Placebo (n=11) | |||
|---|---|---|---|---|
| Primary endpoint: Change from Baseline to week 24 (Log-Transformed) | ||||
| Baseline | Week 24 | Baseline | Week 24 | |
| n | 21 | 21 | 11 | 11 |
| Geometric mean (μM) (CV)c | 354.0 (0.27) | 86.4 (0.50) | 464.7 (0.19) | 426.5 (0.27) |
| Week 24 estimated reduction compared to Baseline (95% CI) | 76.7% (-146.7%, 300.1%) | 0.0% (-234.4%, 232.4%) | ||
| Pegzilarginase Week 24 estimated reduction relative to placebo (95% CI)a | 76.7% (67.1%, 83.5%) | |||
| p-valuea | <0.0001 | |||
| Proportion of subjects achieving target levels in plasma arginine at week 24 | ||||
| Proportion of subjects who achieved guideline recommended target arginine levels (<200 µM) | 19 (90.5%) | 0 (0%) | ||
| Proportion of subjects who achieved normal arginine target levels (defined as <115 µM) | 19 (90.5%) | 0 (0%) | ||
a Based on an MMRM with visit, randomised trial treatment, and interaction between visit and randomised trial treatment as effects and logged Baseline value included as a covariate. Default covariance structure type=unstructured. Week 24 estimated
% reduction was based on geometric mean ratio and accompanying 95% CI;
Abbreviations: CI=confidence interval; CV=coefficient of variation.
Figure 1. Summary of least square mean (95% CI) 168-hour post dose arginine levels (µM) over time in Study 300A double-blind period:
Notes: Medical guideline recommendation for plasma arginine: <200 μM; Normal range defined as 40–115 μM in the clinical trial. Last observation carried forward (LOCF) was used for missing values at Week 24.
Treatment with pegzilarginase also resulted in numerical trends of improvement in mobility relative to placebo after 24 weeks as assessed by GMFM-E, 2MWT and GMFM-D performance (Table 3).
At Week 24, more subjects treated with pegzilarginase met the defined response criteria for arginine and across multiple mobility domains. Eight out of 17 evaluable subjects treated with pegzilarginase met the criteria for response in ≥2 neuromotor function assessments in conjunction with normalisation of plasma arginine levels, with 6 of the responders having no worsening in any assessments. Without treatment with pegzilarginase, no subjects met clinical response criteria on 2 or more clinical outcomes.
Table 3. Analysis of secondary mobility endpoints from Study 300A double-blind period:
| Pegzilarginase (n=21) | Placebo (n=11) | |
|---|---|---|
| GMFM Item E (Change from baseline to week 24) | ||
| n | 20 | 11 |
| Mean (SD) | 4.2 (7.69) | -0.4 (6.2) |
| LS Mean | 4.2 | -0.4 |
| 95% CI for LS Mean | 0.8, 7.6 | -4.9, 4.2 |
| LS Mean Difference (Pegzilarginase – Placebo) (95% CI) | 4.6 (-1.1, 10.2) | |
| 2MWT (Change from baseline to week 24) | ||
| n | 19 | 10 |
| Mean (SD) | 7.3 (30.64) meters | 2.7 (19.66) meters |
| LS Mean | 7.4 | 1.9 |
| 95% CI for LS Mean | -5.0, 19.8 | -15.2, 19.1 |
| LS Mean Difference (Pegzilarginase – Placebo) (95% CI) | 5.5 (-15.6, 26.7) | |
| GMFM Item D (Change from baseline to week 24) | ||
| n | 20 | 10 |
| Mean (SD) | 2.7 (3.88) | 0.4 (0.97) |
| LS Mean | 2.7 | 0.4 |
| LS Mean Difference (Pegzilarginase – Placebo) (95% CI) | 2.3 (-0.4, 4.9) | |
Abbreviations: 2MWT=2-minute walk test; CI=confidence interval; GMFM=Gross Motor Function Measure; LS=least squares, MMRM=mixed model repeated measures; SD=standard deviation; SE=standard error.
Note: Unless noted otherwise, model-based estimates (LS means, differences, 95% CIs, and p-values) are based on an MMRM analysis with visit, randomised trial treatment, and interaction between visit and randomised trial treatment and baseline value as covariates. Default covariance structure type=unstructured.
Long-term treatment in ARG1-D
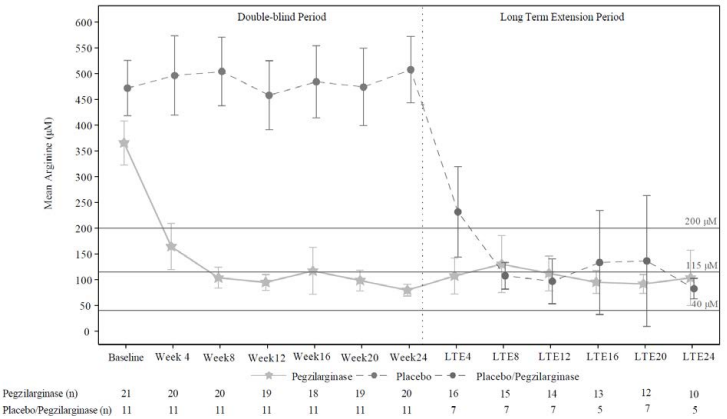
Paediatric and adult subjects who participated in the double-blind period of Study 300A were eligible to continue treatment in an open -label extension period with once weekly pegzilarginase treatment. Thirty-one (n=20 pegzilarginase and n=11 placebo) of the 32 subjects entered the extension period. Subjects previously receiving pegzilarginase were transitioned to subcutaneous administration at the earliest after 8 weeks of intravenous treatment. The median duration of pegzilarginase exposure was 31 weeks (range: 1 to 102 weeks).
During the open-label extension, subjects who previously received pegzilarginase demonstrated sustained improvements in plasma arginine levels, GMFM-E and GMFM-D scores and 2MWT. Subjects randomised initially to placebo and treated with pegzilarginase in the open -label extension period also showed similar reductions from baseline in mean plasma arginine levels (Figure 2).
Figure 2. Summary of mean 168-hour post dose arginine levels (µM) over time in Study 300A double-blind and long-term extension periods:
Notes: 95% confidence interval of mean is displayed; Medical guideline recommendation for plasma arginine: <200 μM; Normal range defined as 40–115 μM in the clinical trial.
Paediatric population
The European Medicines Agency has deferred the obligation to submit the results of studies with Loargys in one or more subsets of the paediatric population in treatment of hyperargininaemia (see section 4.2 for information on paediatric use).
This medicinal product has been authorised under ‘exceptional circumstances’. This means that due to the rarity of the disease it has not been possible to obtain complete information on this medicinal product. The European Medicines Agency will review any new information which may become available every year and this SmPC will be updated as necessary.
5.2. Pharmacokinetic properties
The pharmacokinetic (PK) properties of pegzilarginase were evaluated following intravenous and subcutaneous administration in adults and paediatric subjects with ARG1-D. Population PK analysis has been used to characterise the pharmacokinetics of pegzilarginase.
The following PK parameters at steady state were derived using the final population PK model (Table 4). The final PK model was based on data obtained from 20 female and 17 male subjects, aged 2-31 years old with body weights 12.2-76.7 kg. In the clinical trials, the dose range was 0.015-0.2 mg/kg. Simulated dose in the model was 0.1 mg/kg for 5 weeks.
Table 4. Pharmacokinetic parameters at steady state:
| Pegzilarginase | ||
|---|---|---|
| Intravenous | Subcutaneous | |
| Steady state exposure [Cmax (µg/ml)]* | 2.48 (19.9%) | 0.579 (19.9%) |
| Steady state exposure [AUC0-168 (h*µg/ml)]* | 108 (18.3%) | 61.3 (18.3%) |
| Tmax (h)** | 0.25^ | 34 (22.0-46.0) |
Abbreviations: AUC0-168=area under the concentration-time curve from time 0 to 168 hours; Cmax=maximum observed concentration; t½=half-life; Tmax=time to maximum concentration
* Data displayed are geometric mean and geometric coefficient of variation (%)
** Data displayed as [median (range)]
^ For intravenous dosing, the Tmax corresponds to the time of the first measured PK sample. In these simulations the first PK sample was set at the end of infusion (0.25 h post-dose) for all subjects with no variability.
Simulations were performed for a patient with a body weight of 31kg.
Absorption
Following subcutaneous administration, the mean absolute bioavailability was 57 % and the maximum concentration was reached approximately 34 hours post-dose. Exposure to pegzilarginase increase in an approximately dose-proportional manner with linear PK over a dose range of 0.04 to 0.2 mg/kg intravenous and 0.06 to 0.2 mg/kg subcutaneous. Negligible accumulation was observed after weekly dosing.
Distribution
Pegzilarginase is mainly distributed in the vascular system, with a total volume of distribution of approximately 47 ml/kg, which is similar to human serum volume. The pharmacokinetics was best described with a population-PK model which comprised two-compartments (central and peripheral).
Elimination
Pegzilarginase is a pegylated recombinant human enzyme. To allow once-weekly administration, PEG has been used as a carrier to prolong the half-life of pegzilarginase compared to endogenous arginase. Based on population PK analysis; pegzilarginase has a half-life of approximately 50 hours. The enzyme is expected to be metabolised into small peptides and amino acids by catabolic pathways. Pegzilarginase utilizes a 5 kDa PEG which is eliminated via renal glomerular filtration in patients with normal renal function.
Special populations
Age and sex were not found to be significant covariates once body weight was taken into account. Anti-PEG ADAs were considered an important covariate on clearance, however, this effect was observed with initial doses and it is expected that exposure at steady-state will not be affected.
Renal impairment
Pegzilarginase has not been studied in patients with renal impairment. It cannot be excluded that elimination of PEG is decreased in patients with impaired renal function.
Hepatic impairment
Pegzilarginase has not been studied in patients with hepatic impairment. Changes in the clearance of the enzyme are expected as pegzilarginase is metabolised by catabolic pathways.
Body weight
Overall, body weight had a minimal impact (<20%) on the exposure of pegzilarginase, when dosing is weight based.
5.3. Preclinical safety data
Animal toxicology and/or pharmacology
Dose-dependent and adverse loss of appetite and reductions in body weight gain attributed to marked and sustained arginine depletion below the normal range in normal animals (mice, rats, rabbits and monkeys) was observed in single and repeat dose toxicology studies as well as developmental and reproductive toxicity studies with pegzilarginase. These findings were reversible following cessation of dosing.
In the long-term studies with pegzilarginase, male reproductive toxicities were noted in a single species, healthy juvenile rats. The principal adverse findings at dose levels ≥0.3 mg/kg, included decreased weights of testes, seminal vesicles, epididymides and prostate, atrophy was observed in the seminiferous tubules. The male rat organ weight findings were reversible. Histopathology confirmed findings in the testes and epididymides, which were not reversible in the recovery period of 6 weeks; however, it is worth noting that the normal sperm cycle is 9 weeks. These effects could be due to exaggerated pharmacology in normal animals with normal circulating arginine levels at baseline. However, the relevance for humans is unclear.
Reproductive and developmental toxicology
Studies conducted with pegzilarginase in rats and rabbits with normal circulating arginine levels demonstrated maternal reproductive toxicity that is associated with sustained decreases in plasma arginine concentrations below the normal range during gestation. Toxicities associated with the prolonged exaggerated pharmacology in pregnant animals were decreased maternal body weights, food consumption, and mean gravid uterine weights and associated secondary fetal growth retardation.
In pre- and postnatal development toxicology studies in rats with normal circulating arginine levels, male rat offspring of nursing animals dosed with 1 mg/kg pegzilarginase (approximately 7 times human exposure based on AUC) revealed deficits possibly due to secondary effects related to exaggerated pharmacology in animals with normal circulating arginine levels (see section 4.6).
Fertility
During fertility assessments conducted in normal animals with normal circulating arginine levels, male rats dosed at 1 mg/kg showed decreased sperm production and motility. Additionally, in naïve female rats paired with males treated at 1 mg/kg/dose for 8 weeks prior to mating, pegzilarginase-related effects included a significant reduction in uterine implantation sites and increased pre-implantation loss.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.