LOQTORZI Solution for injection Ref.[107258] Active ingredients: Toripalimab
Source: FDA, National Drug Code (US) Revision Year: 2024
12.1. Mechanism of Action
Binding of the PD-1 ligands, PD-L1 and PD-L2, to the PD-1 receptor found on T cells, inhibits T cell proliferation and cytokine production. Upregulation of PD-1 ligands occurs in some tumors and signaling through this pathway can contribute to inhibition of active T-cell immune surveillance of tumors. Toripalimab-tpzi is a humanized IgG4 monoclonal antibody that binds to the PD-1 receptor and blocks its interaction with PD-L1 and PD-L2, releasing PD-1 pathway-mediated inhibition of the immune response, including the anti-tumor immune response. In syngeneic mouse tumor models, blocking PD-1 activity resulted in decreased tumor growth.
12.2. Pharmacodynamics
The toripalimab-tpzi exposure-response relationships and time course of pharmacodynamic response are not fully characterized.
12.3. Pharmacokinetics
Toripalimab-tpzi pharmacokinetic parameters are presented as geometric mean (coefficient of variation [CV]%) unless otherwise noted. Toripalimab-tpzi concentrations increased in non-linearly over the dose range of 0.3 to 10 mg/kg every two weeks (0.1 to 3.3 times the approved recommended 3 mg/kg dosage in a 64 kg patient). Steady state was reached by Week 7. The mean accumulation ratio was approximately 1.4 for maximum concentration (Cmax) and 1.9 for area under the serum concentration curve (AUC) following multiple doses at the approved recommended dosages of 240 mg Q3W in combination with cisplatin and gemcitabine and 3 mg/kg Q2W as monotherapy.
Distribution
The mean volume of distribution at steady state (Vss) of toripalimab-tpzi was 3.7 L (27%).
Elimination
The mean clearance (CL) was 14.9 mL/h (31%) after the first dose and 9.5 mL/h (36%) at steady state. The mean terminal half-life (t1/2) (± standard deviation) was 10 ± 1.5 days after the first dose and 18 ± 9.4 days at steady state.
Metabolism
Toripalimab-tpzi is expected to be metabolized into small peptides by catabolic pathways.
Specific Populations
No clinically significant differences in the pharmacokinetics were observed based on age (21 to 85 years), body weight (32 to 164 kg), sex, race (White and Asian), concomitant chemotherapy, mild renal impairment (creatinine clearance [CLcr] 60 to 89 mL/min), mild hepatic impairment (total bilirubin >1 to 1.5 times ULN with any AST or total bilirubin ≤ ULN with AST > ULN), tumor burden and primary cancer.
The effect of moderate (total bilirubin >1.5 to 3 times ULN and any AST) or severe (total bilirubin >3 times ULN and any AST) hepatic impairment or of moderate (CLcr 30 to 59 mL/min) or severe (CLcr 15 to 29 mL/min) renal impairment on the pharmacokinetics of toripalimab-tpzi has not been studied.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed to test the potential of toripalimab-tpzi for carcinogenicity or genotoxicity.
Fertility studies have not been conducted with toripalimab-tpzi. In 4-week and 26-week repeat-dose toxicology studies in sexually mature cynomolgus monkeys, there were no adverse or notable effects in the male and female reproductive organs.
13.2. Animal Toxicology and/or Pharmacology
In animal models, inhibition of PD-1/PD-L1 signaling increased the severity of some infections and enhanced inflammatory responses. Mycobacterium tuberculosis-infected PD-1 knockout mice exhibit markedly decreased survival compared with wild-type controls, which correlated with increased bacterial proliferation and inflammatory responses in these animals. PD-1 blockade using a primate anti-PD1 antibody was also shown to exacerbate M. tuberculosis infection in rhesus macaques. PD-1 and PD-L1 knockout mice and mice receiving PD-L1 blocking antibody have also shown decreased survival following infection with lymphocytic choriomeningitis virus.
14. Clinical Studies
14.1 First-line Treatment of Metastatic or Recurrent, Locally Advanced NPC with Cisplatin and Gemcitabine
The efficacy of LOQTORZI in combination with cisplatin and gemcitabine was investigated in JUPITER-02 (NCT03581786), a randomized, multicenter, single region, double-blind, placebo-controlled trial in 289 patients with metastatic or recurrent, locally advanced NPC who had not received previous systemic chemotherapy for recurrent or metastatic disease. Patients with recurrent NPC after treatment with curative intent were required to have an interval of at least 6 months between the last dose of radiotherapy or chemotherapy and recurrence. Patients with autoimmune disease, other than stable hypothyroidism or Type I diabetes, and patients who required systemic immunosuppression were ineligible.
Randomization was stratified according to Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) (0 versus 1) and disease stage (recurrent versus metastatic). Patients were randomized (1:1) to receive one of the following treatments:
- LOQTORZI 240 mg intravenously every 3 weeks in combination with cisplatin 80 mg/m2 on Day 1 every 3 weeks gemcitabine 1000 mg/m² on Days 1 and 8 for up to 6 cycles, followed by LOQTORZI 240 mg once every 3 weeks, or
- Placebo intravenously every 3 weeks in combination with cisplatin 80 mg/m² on Day 1 every 3 weeks and gemcitabine 1000 mg/m² on Days 1 and 8 for up to 6 cycles, followed by placebo once every 3 weeks.
Treatment with LOQTORZI or placebo continued until disease progression per RECIST v1.1, unacceptable toxicity, or a maximum of 2 years. Administration of LOQTORZI was permitted beyond radiographic progression if the patient was deriving benefit as assessed by the investigator. Tumor assessments were performed every 6 weeks for the first 12 months and every 9 weeks thereafter. The main efficacy outcome measure was Blinded Independent Review Committee (BIRC)-assessed progression-free survival (PFS) according to RECIST v1.1. Additional efficacy outcome measures include BIRC-assessed overall response rate (ORR) and overall survival (OS).
The study population characteristics were: median age of 48 years (range: 19 to 72), 4.8% age 65 or older, 83% male, 100% Asian, and 57% had ECOG PS of 0. Eighty-six percent of patients had metastatic disease at study entry. Histological subtypes of NPC included 98% non-keratinizing, 1% keratinizing squamous cell carcinoma, and 1% did not have the subtype identified.
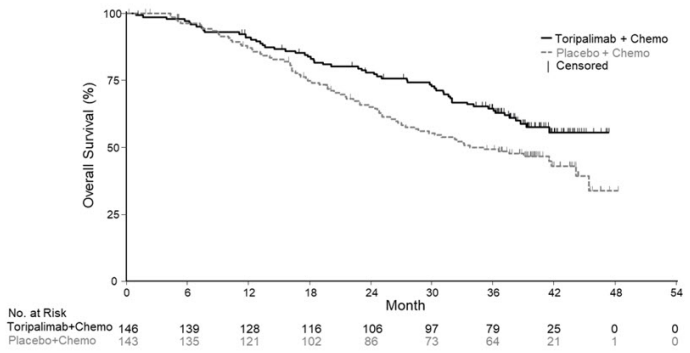
Efficacy results of the pre-specified interim analysis of PFS and final analysis of OS are summarized in Table 7 and Figure 1 below. The trial demonstrated statistically significant improvements in BIRC-assessed PFS, ORR and OS for patients randomized to LOQTORZI in combination with cisplatin/gemcitabine compared to cisplatin and gemcitabine with placebo.
Table 7. Efficacy Results in JUPITER-02:
| Endpoints | LOQTORZI Cisplatin/Gemcitabine N =146 | Placebo Cisplatin/Gemcitabine N =143 |
|---|---|---|
| BIRC-Assessed Progression-free Survival* | ||
| Number of Events, n (%) | 49 (34) | 79 (55) |
| Median, months (95% CI) | 11.7 (11.0, NE) | 8.0 (7.0, 9.5) |
| Hazard Ratio† (95% CI) | 0.52 (0.36, 0.74) | |
| p-value‡ | 0.0003 | |
| BIRC-Assessed Overall Response Rate* | ||
| ORR, % (95% CI) | 77 (70, 84) | 66 (58, 74) |
| Complete Response Rate (%) | 19 | 11 |
| Partial Response Rate (%) | 58 | 55 |
| p-value§ | 0.0353 | |
| BIRC-Assessed Duration of Response | ||
| Median, months (95% CI) | 10.0 (8.8, NE) | 5.7 (5.4, 6.8) |
| Overall Survival¶ | ||
| Number of Deaths, n (%) | 57 (39) | 76 (53) |
| Median, months (95% CI) | NR (38.7, NE) | 33.7 (27.0, 44.2) |
| Hazard Ratio† (95% CI) | 0.63 (0.45, 0.89) | |
| p-value# | 0.0083 | |
BIRC=blinded independent review committee; CI= confidence interval; NR=Not Reached; NE=Not estimable.
* PFS and ORR results are based on the pre-specified interim analysis with data cutoff of May 30, 2020.
† Based on the stratified Cox proportional-hazards model using the stratification factors at randomization, ECOG performance status and disease stage.
‡ Two-sided p-value, based on the stratified log-rank test, as compared with an alpha boundary of 0.010.
§ Two-sided p-value, based upon Cochran-Mantel-Haenszel test.
¶ OS results are based on the final analysis with a data cutoff of November 18, 2022.
# Two-sided p-value, based on the stratified log-rank test, as compared with an alpha boundary of 0.049995.
Figure 1. Kaplan-Meier Curves of Overall Survival for JUPITER-02:
14.2 Previously Treated Unresectable or Metastatic NPC
The efficacy of LOQTORZI was investigated in POLARIS-02 (NCT 02915432), an open-label, multicenter, multicohort trial conducted in a single country. The trial included a total of 172 patients with unresectable or metastatic NPC who had received prior platinum-based chemotherapy for treatment of recurrent or metastatic NPC or had disease progression within 6 months of completion of platinum-based chemotherapy administered as neoadjuvant, adjuvant, or definitive chemoradiation treatment for locally advanced disease. Key exclusion criteria included previous treatment with an anti-PD-(L)1 antibody; active autoimmune disease or other medical conditions requiring immunosuppressive therapy. Patients received LOQTORZI 3 mg/kg intravenously every 2 weeks until disease progression per RECIST v1.1 or unacceptable toxicity. Tumor response assessments were performed every 8 weeks for the first year and every 12 weeks thereafter. The major efficacy outcome measures were confirmed ORR and duration of response (DOR) as assessed by a Blinded Independent Review Committee (BIRC) using RECIST v1.1.
The median age was 45 years (range: 22 to 68), 4.1% age 65 or older, 83% male, 100% Asian, and Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) of 0 (37%). Patients had received a median of 2 prior systemic therapies for recurrent/metastatic disease (range: 1-13). Ninety-five percent of patients had metastatic disease, 95% had non-keratinizing NPC, 2.9% had keratinizing squamous cell carcinoma and 1.7% did not have the subtype identified.
Efficacy results for POLARIS-02 are summarized in Table 8 below.
Table 8. Efficacy Results for POLARIS-02:
| Endpoint | LOQTORZI (N=172) |
|---|---|
| BIRC-Assessed Overall Response Rate* | |
| Overall Response Rate, (95 CI) | 21 (15, 28) |
| Complete Response Rate, % | 2.3 |
| Partial Response Rate, % | 19 |
| BIRC-Assessed Duration of Response (DOR) | (N = 36) |
| Median, months (95% CI) | 14.9 (10.3, NE) |
| Patients with DOR ≥6 months†, n (%) | 30 (83%) |
| Patients with DOR ≥12 months†, n (%) | 14 (39%) |
CI=confidence interval. n=number. NE = not estimable.
BIRC=blinded independent review committee
* Confirmed overall response rate assessed by BIRC
† Based on observed duration of response
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.