LUTATHERA Solution for infusion Ref.[10985] Active ingredients: Lutetium ¹⁷⁷Lu oxodotreotide
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: Advanced Accelerator Applications, 20 rue Diesel, 01630 Saint Genis Pouilly, France
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Other therapeutic radiopharmaceuticals
ATC code: V10XX04
Mechanism of action
Lutetium (177Lu) oxodotreotide has a high affinity for subtype 2 somatostatin receptors (sst2). It binds to malignant cells which overexpress sst2 receptors. Lutetium-177 (177Lu) is a βemitting radionuclide with a maximum penetration range in tissue of 2.2 mm (mean penetration range of 0.67 mm), which is sufficient to kill targeted tumour cells with a limited effect on neighbouring normal cells.
Pharmacodynamic effects
At the concentration used (about 10 μg/mL in total, for both free and radiolabeled forms), the peptide oxodotreotide does not exert any clinically relevant pharmacodynamic effect.
Clinical efficacy and safety
NETTER-1 phase III study was a multicentre stratified, open labelled, randomized, comparator-controlled, parallel-group study comparing treatment with Lutathera (4 doses of 7,400 MBq every 8 weeks) co-administered with amino acid solution plus best supportive care (BSC; octreotide long acting release [LAR] 30 mg every 4 weeks for symptoms control, replaced by short acting octreotide in the 4 weeks interval before Lutathera administration) to high dose octreotide LAR (60 mg every 4 weeks) in patients with inoperable, progressive, somatostatin receptor positive, midgut carcinoid tumours. The primary endpoint for the study was progression-free survival (PFS) evaluated by response evaluation criteria in solid tumours (RECIST 1.1), based on independent radiology assessment. Secondary endpoints included objective response rate (ORR), overall survival (OS), time to tumour progression (TTP), safety and tolerability of the medicinal product and quality of life (QoL). Two hundred twenty-nine (229) patients have been randomized to receive either Lutathera (n=116) or high dose 60 mg octreotide LAR (n=113). Demographics as well as patients and disease characteristics were well balanced between groups with a median age of 64 years and 82.1% Caucasian in the general population.
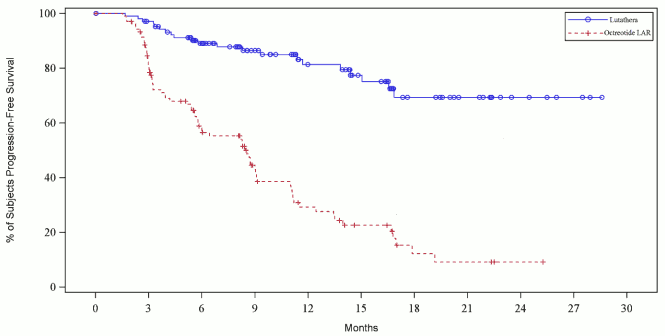
At the time of final per-protocol PFS statistical analysis (cut–off date 24 July 2015), the number of centrally confirmed disease progressions or deaths was 21 events in the Lutathera arm and 70 events in the octreotide LAR arm (Table 6). PFS differed significantly (p<0.0001) between the treatment groups. The median PFS for Lutathera was not reached at the time of analysis whereas the one of octreotide LAR was 8.5 months. The hazard ratio for Lutathera was 0.18 (95% CI: 0.11-0.29), indicating 82% reduction in the risk for a patient to progress or die under Lutathera compared to octreotide LAR.
Table 6. PFS observed in the NETTER-1 phase III study in patients with progressive midgut carcinoid tumour – cut–off date 24 July 2015 (full analyses set (FAS), N=229):
| Treatment | ||
|---|---|---|
| Lutathera | Octreotide LAR | |
| N | 116 | 113 |
| Patients with events | 21 | 70 |
| Censored patients | 95 | 43 |
| Median months (95%-CI) | Not reached | 8.5 (5.8; 9.1) |
| p-value of Log-rank test | <0.0001 | |
| Hazard ratio (95%-CI) | 0.177 (0.108; 0.289) | |
N: number of patients, CI: confidence interval.
The PFS Kaplan-Meier graph for the full analysis set (FAS) at the cut–off date 24 July 2015 is depicted in Figure 3.
Figure 3. PFS Kaplan Meier curves of patients with progressive midgut carcinoid tumour – cut-off date 24 July 2015 (NETTER-1 phase III study; FAS, N=229):
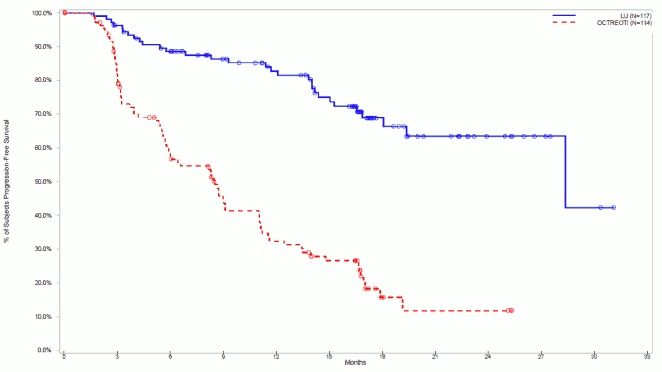
At the cut-off date for post-hoc statistical analysis (30 June 2016), the number of centrally confirmed disease progressions or deaths was 30 events in the Lutathera arm and 78 events in the octreotide LAR arm (Table 7). PFS differed significantly (p<0.0001) between the treatment groups. The median PFS for Lutathera was 28.4 months whereas the one of octreotide LAR was 8.5 months. The hazard ratio for Lutathera was 0.21 (95% CI: 0.14-0.33), indicating 79% reduction in the risk for a patient to progress or die under Lutathera compared to octreotide LAR.
Table 7. PFS observed in the NETTER-1 phase III study in patients with progressive midgut carcinoid tumour - cut-off date 30 June 2016 (full analyses set (FAS), N=231):
| Treatment | ||
|---|---|---|
| Lutathera | Octreotide LAR | |
| N | 117 | 114 |
| Patients with events | 30 | 78 |
| Censored patients | 87 | 36 |
| Median months (95%-CI) | 28.4 (28.4; NE) | 8.5 (5.8; 11.0) |
| p-value of Log-rank test | <0.0001 | |
| Hazard ratio (95%-CI) | 0.214 (0.139; 0.331) | |
N: number of patients, CI: confidence interval.
The PFS Kaplan-Meier graph for the full analysis set (FAS) at the cut-off date 30 June 2016 is depicted in Figure 4.
Figure 4. PFS Kaplan Meier curves of patients with progressive midgut carcinoid tumour – cut-off date 30 June 2016 (NETTER-1 phase III study; FAS, N=231):
With respect to overall survival OS, at the time of interim analysis (24 July 2015), there were 17 deaths in the Lutathera arm and 31 in octreotide LAR 60 mg arm and the hazard ratio was 0.459 in favour of Lutathera, but did not reach the level of significance for interim analysis (HR 99.9915% CI: 0.140, 1.506). OS median was 27.4 months in octreotide LAR arm and was not reached in Lutathera arm. An update conducted about one year after (30 June 2016) showed similar trend with 28 deaths in the Lutathera arm and 43 in octreotide LAR 60 mg arm, an HR of 0.536, and a median OS of 27.4 months in octreotide LAR arm and still not reached in Lutathera arm. The final OS analysis is foreseen after 158 cumulative deaths.
Health Related Quality of Life (HRQOL) was assessed using the European Organisation for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30) (generic instrument) and its neuroendocrine tumour module (EORTC QLQ-GI.NET-21). The results indicate an improvement in the overall global health-related quality of life up to week 84, for patients on Lutathera treatment as compared to patients on Octreotide LAR.
Erasmus phase I/II study was a monocentric single arm open-label study to evaluate the efficacy of Lutathera (7,400 MBq administered for 4 times every 8 weeks) co-administered with amino acid solution in patients with somatostatin receptor positive tumours. The mean age of patients enrolled in the study was 60 years. Most patients were Dutch (811) with the remaining (403) residents of various European and non-European countries. The main analysis has been conducted on 811 Dutch patients with different somatostatin receptor positive tumour types. The ORR (including complete response (CR) and partial response (PR) according to RECIST criteria) and duration of response (DoR) for the FAS Dutch population with gastroenteropancreatic (GEP) and bronchial NETs (360 patients) as well as per tumour type are presented in Table 8.
Table 8. Best response, ORR and DoR observed in the Erasmus phase I/II study in Dutch patients with GEP and bronchial NETs – (FAS, N=360):
| Tumour type | N | CR | PR | SD | ORR | DoR (months) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % | n | % | N | % | n | % | 95%CI | Median | 95%CI | ||||
| All* | 360 | 11 | 3% | 151 | 42% | 183 | 51% | 162 | 45% | 40% | 50% | 16.3 | 12.2 | 17.8 |
| Bronchial | 19 | 0 | 0% | 7 | 37% | 11 | 58% | 7 | 37% | 16% | 62% | 23.9 | 1.7 | 30.0 |
| Pancreatic | 133 | 7 | 5% | 74 | 56% | 47 | 35% | 81 | 61% | 52% | 69% | 16.3 | 12.1 | 21.8 |
| Foregut** | 12 | 1 | 8% | 6 | 50% | 4 | 33% | 7 | 58% | 28% | 85% | 22.3 | 0.0 | 38.0 |
| Midgut | 183 | 3 | 2% | 58 | 32% | 115 | 63% | 61 | 33% | 27% | 41% | 15.3 | 10.5 | 17.7 |
| Hindgut | 13 | 0 | 0% | 6 | 46% | 6 | 46% | 6 | 46% | 19% | 75% | 17.8 | 6.2 | 29.9 |
CR = Complete response; PR = Partial response; SD = Stable disease; ORR = Objective response (CR+PR); DoR = Duration of response
* Includes Foregut, Midgut and Hindgut; **Foregut NETs other than bronchial and pancreatic
The overall median PFS and OS for the FAS Dutch population with GEP and bronchial NETs (360 patients) as well as per tumour type are presented in Table 9.
Table 9. PFS and OS observed in the Erasmus phase I/II study in Dutch patients with GEP and bronchial NET – (FAS, N=360):
| PFS Time (months) | OS Time (months) | ||||||
|---|---|---|---|---|---|---|---|
| Median | 95%CI | Median | 95%CI | ||||
| All* | 360 | 28.5 | 24.8 | 31.4 | 61.2 | 54.8 | 67.4 |
| Bronchial | 19 | 18.4 | 10.4 | 25.5 | 50.6 | 31.3 | 85.4 |
| Pancreatic | 133 | 30.3 | 24.3 | 36.3 | 66.4 | 57.2 | 80.9 |
| Foregut** | 12 | 43.9 | 10.9 | 21.3 | |||
| Midgut | 183 | 28.5 | 23.9 | 33.3 | 54.9 | 47.5 | 63.2 |
| Hindgut | 13 | 29.4 | 18.9 | 35.0 | |||
PFS = Progression free survival; OS = Overall survival
* Includes Foregut, Midgut and Hindgut; **Foregut NETs other than bronchial and pancreatic
In the Erasmus phase I/II study 188 patients (52%) received and 172 (48%) did not receive concomitant octreotide LAR during Lutathera treatment. No statistically significant difference in PFS was observed between the subgroup of patients who did not receive octreotide LAR (25.4 months [95% CI 22.8-30.6]) versus the subgroup who did receive concomitant treatment with octreotide LAR (30.9 months [95% CI 25.6-34.8]) (p=0.747).
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with Lutathera in all subsets of the paediatric population in the treatment of GEP-NETs (excluding neuroblastoma, neuroganglioblastoma, phaeochromocytoma). See section 4.2.
5.2. Pharmacokinetic properties
Absorption
The medicinal product is administered intravenously and is immediately and completely bioavailable.
Organ uptake
At 4 hours after administration, the distribution pattern of lutetium (177Lu) oxodotreotide shows a rapid uptake in kidneys, tumour lesions, liver and spleen, and in some patients in the pituitary gland and in the thyroid. The co-administration of amino acid solution decreases the kidney uptake, enhancing the elimination of radioactivity (see section 4.4). Biodistribution studies show that lutetium (177Lu) oxodotreotide is rapidly cleared from the blood.
An analysis performed with human plasma to determine the extent of plasma protein binding of non-radioactive compound (lutetium (175Lu) oxodotreotide) showed that about 50% of the compound is bound to plasmatic proteins. Transchelation of lutetium from lutetium (175Lu) oxodotreotide into serum proteins has not been observed.
Biotransformation
There is evidence, from the analysis of urine samples of 20 patients included in the NETTER-1 phase III Dosimetry, pharmacokinetic and ECG substudy, that lutetium (177Lu) oxodotreotide is poorly metabolized and is excreted mainly as intact compound by renal route.
The high performance liquid chromatography (HPLC) analyses performed on urine samples collected up to 48 hours post infusion showed lutetium (177Lu) oxodotreotide radiochemical purity close to 100% in most of the analysed samples (with lowest radiochemical purity value being greater than 92%), indicating that the compound is eliminated in urine mainly as intact compound.
This evidence confirms what has been previously observed in the Erasmus phase I/II study, in which HPLC analysis of a urine specimen collected 1 hour post administration of lutetium (177Lu) oxodotreotide from one patient receiving 1.85 MBq of lutetium (177Lu) oxodotreotide indicated that the main portion (91%) was excreted unchanged.
These finding are supported by in vitro metabolism data in human hepatocytes, in which no metabolic degradation of lutetium (175Lu) oxodotreotide was observed.
Elimination
Based on the data collected during the Erasmus phase I/II and NETTER-1 phase III studies, lutetium (177Lu) oxodotreotide is primarily eliminated by renal excretion: about 60% of the medicinal product is eliminated in the urine within 24 hours, and about 65% within 48 hours following the administration.
Elderly
The pharmacokinetics profile in elderly patients (≥75 years) has not been established. No data are available.
5.3. Preclinical safety data
Toxicological studies with rats have demonstrated that a single intravenous injection of up to 4,550 MBq/kg was well tolerated and no deaths were observed. When testing the cold compound (non-radioactive lutetium (175Lu) oxodotreotide) as a single intravenous injection in rats and dogs at doses up to 20,000 µg/kg (rats) and 3,200 µg/kg (dogs), the compound was well tolerated in both species and no deaths were observed. Toxicity with four repeated administrations, once every 2 weeks, of 1,250 µg/kg of the cold compound in rats and 80 µg/kg in dogs was not observed. This medicinal product is not intended for regular or continuous administration. Mutagenicity studies und long-term carcinogenicity studies have not been carried out.
Non-clinical data on the cold compound (non-radioactive lutetium (175Lu) oxodotreotide) reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.