LUVOX Tablet Ref.[50292] Active ingredients: Fluvoxamine
Source: Marketing Authorisation Holder Revision Year: 2022 Publisher: Viatris Pty Ltd, Level 1, 30 The Bond, 30-34 Hickson Road, Millers Point NSW 2000, www.viatris.com.au, Phone: 1800 274 276
Product name and form
LUVOX Fluvoxamine maleate.
| Pharmaceutical Form |
|---|
|
UVOX (fluvoxamine maleate) 50 mg: Round, biconvex, scored, white to off-white film coated tablet inscribed on one face with 291 on either side of the score line and other face plain. LUVOX (fluvoxamine maleate) 100 mg: Oval, biconvex, scored, white to off-white film coated tablet inscribed on one face with 313 on either side of the score line and other face plain. |
Qualitative and quantitative composition
Each tablet of LUVOX contains 50 mg or 100 mg of fluvoxamine maleate.
Fluvoxamine is a member of a class of antidepressant agents known as selective serotonin reuptake inhibitors (SSRI). It is chemically unrelated to the tricyclic antidepressants, and to other serotonin reuptake inhibitors as it is a monocyclic compound.
For the full list of excipients, see Section 6.1 LIST OF EXCIPIENTS.
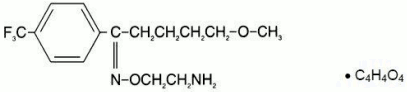
Chemical Structure
MW = 434.4
Fluvoxamine maleate is chemically identified as (E)-5-methoxy-4’-trifluoromethylvalerophenone 0-2-aminoethyloxime maleate.
Fluvoxamine maleate is a white to slightly off-white, odourless, crystalline powder, sparingly soluble in water, freely soluble in ethanol and chloroform, practically insoluble in diethylether. It has a partition coefficient (noctanol/water, own pH) P=38.7.
CAS Number: 61718-82-9
| Active Ingredient |
|---|
|
The mechanism of action of fluvoxamine is thought to be related to selective serotonin re-uptake inhibition in brain neurones. There is minimum interference with noradrenergic processes. Receptor binding studies have demonstrated that fluvoxamine has negligible binding capacity to alpha adrenergic, beta adrenergic, histaminergic, muscarine cholinergic, dopaminergic or serotonergic receptors. |
| List of Excipients |
|---|
|
The inactive ingredients are: mannitol, maize starch, pregelatinised potato starch, sodium stearylfumarate, colloidal anhydrous silica, hypromellose, macrogol 6000, purified talc, titanium dioxide. |
Pack sizes and marketing
LUVOX tablets are available in blister packs (PVC/PVDC/Al).
50 mg tablets: packs of 60^*^, 30 and 10 (sample pack).
100 mg tablets: packs of 30 and 10 (sample pack).
* Not distributed in Australia
Australian Register of Therapeutic Goods (ARTG)
AUST R 57632 – LUVOX fluvoxamine maleate 50mg tablet blister pack.
AUST R 57633 – LUVOX fluvoxamine maleate 100mg tablet blister pack.
Marketing authorization holder
Viatris Pty Ltd, Level 1, 30 The Bond, 30-34 Hickson Road, Millers Point NSW 2000, www.viatris.com.au, Phone: 1800 274 276
Marketing authorization dates and numbers
1/04/1997
Drugs
| Drug | Countries | |
|---|---|---|
| LUVOX | Australia, Brazil, Canada, Ecuador, Japan, Mexico, New Zealand, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.