Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: AstraZeneca AB, SE-151 85 Södertälje, Sweden
Pharmacotherapeutic group: antineoplastic agents, other antineoplastic agents
ATC code: L01XX46
Olaparib is a potent inhibitor of human poly (ADP-ribose) polymerase enzymes (PARP-1, PARP-2 and PARP-3), and has been shown to inhibit the growth of selected tumour cell lines in vitro and tumour growth in vivo either as a standalone treatment or in combination with established chemotherapies.
PARPs are required for the efficient repair of DNA single strand breaks and an important aspect of PARP-induced repair requires that after chromatin modification, PARP auto-modifies itself and dissociates from the DNA to facilitate access for base excision repair (BER) enzymes. When olaparib is bound to the active site of DNA-associated PARP it prevents the dissociation of PARP and traps it on the DNA, thus blocking repair. In replicating cells this leads to DNA double strand breaks (DSBs) when replication forks meet the PARP-DNA adduct. In normal cells, homologous recombination repair (HRR), which requires functional BRCA1 and 2 genes, is effective at repairing these DNA DSBs. In the absence of functional BRCA1 or 2, DNA DSBs cannot be repaired via HRR. Instead, alternative and error-prone pathways are activated, such as the non-homologous end joining (NHEJ) pathway, leading to increased genomic instability. After a number of rounds of replication, genomic instability can reach insupportable levels and result in cancer cell death, as cancer cells have a high DNA damage load relative to normal cells.
In BRCA-deficient in vivo models, olaparib given after platinum treatment resulted in a delay in tumour progression and an increase in overall survival compared to platinum treatment alone.
Local or central testing of blood or tumour samples for BRCA1/2 mutations has been used in different studies. Depending on the test used and the international classification consensus, the BRCA1/2 mutations have been classified as deleterious/suspected deleterious or pathogenic/likely pathogenic. Genetic testing should be conducted by an experienced laboratory using a validated test.
The safety and efficacy of olaparib as a maintenance therapy in the treatment of platinum-sensitive relapsed (PSR) high grade serous ovarian, including fallopian tube or primary peritoneal cancer patients, following treatment with two or more platinum-containing regimens, were studied in a Phase II randomised, double-blind, placebo-controlled trial (study 19). The study compared the efficacy of olaparib maintenance treatment taken until progression with no maintenance treatment in 265 (136 olaparib and 129 placebo) PSR serous ovarian cancer patients who were in response (CR [complete response] or PR [partial response]) confirmed as per RECIST and/or as per CA-125 criteria as defined by Gynecologic Cancer InterGroup (GCIG) (at least a 50% reduction in CA-125 levels from the last pre-treatment sample, confirmed 28 days later) following completion of two or more previous platinum-containing chemotherapy. The primary endpoint was PFS (progression-free survival) based on investigator assessment using RECIST 1.0. Secondary efficacy endpoints included OS (overall survival), DCR (disease control rate) defined as confirmed CR/PR + SD (stable disease), HRQoL (health related quality of life), and disease related symptoms. Exploratory analyses of time to first subsequent therapy or death (TFST) and time to second subsequent therapy or death (TSST- an approximation of PFS2) were also performed.
Only PSR patients with partially platinum-sensitive disease (platinum-free interval of 6 to 12 months) and patients with platinum-sensitive disease (platinum-free interval of >12 months) who were in response following completion of last platinum-based chemotherapy were enrolled. Patients could not have received prior olaparib or other PARP inhibitor treatment. Patients could have received prior bevacizumab, except in the regimen immediately prior to randomisation. Retreatment with olaparib was not permitted following progression on olaparib. Most patients were ECOG performance status 0 (77%), there are no data in patients with performance status 2 to 4.
Patients were randomised into the study a median of 40 days after completing their final platinum chemotherapy. They received an average of 3 previous chemotherapy regimens (range 2-11) and 2.6 previous platinum-containing chemotherapies (range 2-8). Platinum free interval was >12 months in 60% and >6-12 months in 40% of the patients. Response to prior platinum chemotherapy was complete in 45% and partial in 55% of the patients. In the olaparib and placebo arms, 6% and 5% of patients had prior bevacizumab, respectively.
Patients in the olaparib group continued to receive treatment longer than those in the placebo group. A total of 32 (23.5%) patients received treatment for ≥2 years in the olaparib group compared with 5 (3.9%) patients in the placebo group. A total of 18 (13.2%) patients received treatment for ≥5 years in the olaparib group compared with 1 (0.8%) patient in the placebo group.
The study met its primary objective demonstrating a statistically significant improvement in PFS for olaparib compared with placebo in the overall population with a hazard ratio (HR) of 0.35 (95% CI 0.25-0.49; p<0.00001; median 8.4 months olaparib versus 4.8 months placebo). At the final OS analysis (data cut off [DCO] 9 May 2016) at 79% maturity, the HR comparing olaparib with placebo was 0.73 (95% CI 0.55-0.95; p=0.02138 [did not meet pre-specified significance level of <0.0095]; median 29.8 months olaparib versus 27.8 months placebo).
Pre-planned subgroup analysis by BRCA-mutation status identified patients with BRCA-mutated ovarian cancer (n=136, 51.3%) as the subgroup that derived the greatest clinical benefit from olaparib maintenance monotherapy. Enrolment did not require evidence of BRCA1/2 mutation (BRCA mutation status for some patients was determined retrospectively). There are limited data in patients with somatic BRCA mutated tumours; 10 patients in the olaparib arm and 10 patients in the placebo arm were defined as having somatic BRCA1/2 mutation. There was no strategy for multiple testing in place for the sub-group analyses.
In BRCA-mutated patients (n=136) there was a statistically significant improvement in PFS, TFST and TSST. The median PFS improvement was 6.9 months over placebo for olaparib-treated patients (HR 0.18; 95% CI 0.10-0.31; p<0.00001; median 11.2 months versus 4.3 months). The investigator assessment of PFS was consistent with a blinded independent central radiological review of PFS. At the final analysis (DCO 9 May 2016), the time from randomisation to start of first subsequent therapy or death (TFST) was 9.4 months longer for olaparib-treated patients (HR 0.33; 95% CI 0.22–0.49; p<0.00001; median 15.6 months versus 6.2 months). The time from randomisation to start of second subsequent therapy or death (TSST) was 6.1 months longer for olaparib-treated patients (HR 0.43; 95% CI 0.29-0.64; p=0.00003; median 21.4 months versus 15.3 months). For the secondary endpoint of OS, the HR for olaparib versus placebo was 0.62 (95% CI 0.42-0.93; p=0.02140; median 34.9 months versus 30.2 months) (Table 2). In the olaparib-treated group, 28.4% of patients remained on treatment for ≥2 years and 14.9% for ≥5 years. In the placebo-treated group, 8.1% of patients remained on treatment for ≥2 years and 1.6% for ≥5 years. Within the BRCA-mutated population the disease control rate at 24 weeks was 57% and 24% for patients in the olaparib and placebo groups, respectively.
No statistically significant differences were observed between olaparib and placebo in patient reported symptoms or HRQoL as measured by improvement and worsening rates in the FACT/NCCN Ovarian Symptom Index (FOSI), Trial Outcome Index (TOI) and Functional Analysis of Cancer Therapy–Ovarian total score (FACT-O total).
The key efficacy findings from Study 19 for BRCA-mutated patients are presented in Table 2, and Figures 1 and 2.
Table 2. Summary of key efficacy findings for patients with BRCA-mutated PSR ovarian cancer in Study 19:
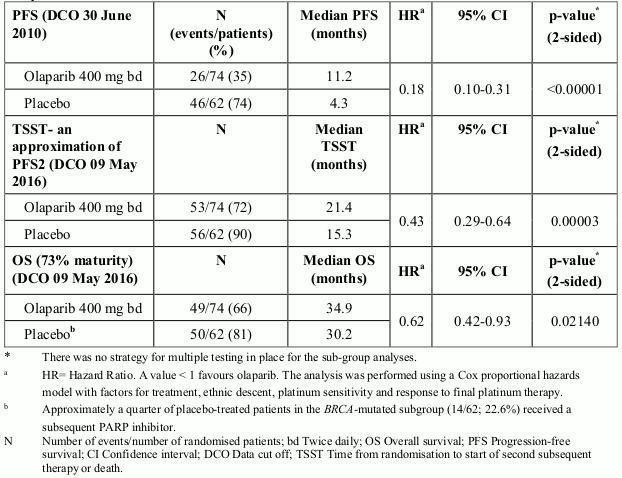
Figure 1. Study 19: Kaplan-Meier plot of PFS in BRCA-mutated patients (53% maturity-investigator assessment):
Figure 2. Study 19: Kaplan-Meier plot of OS in BRCA-mutated patients (73% maturity):
In Study 19, 20 patients were identified with a somatic tumour BRCA mutation (a mutation in the tumour but wildtype in the germline). The limited data for these somatic tumour BRCA (sBRCA) mutated patients show that fewer patients on olaparib reported progression events or death events compared with placebo (Table 3).
Table 3. Summary of progression-free survival and overall survival: sBRCA mutated population in Study 19:
| N events/patients (%) | |
|---|---|
| PFS | |
| Olaparib 400 mg bd | 3/10 (30%) |
| Placebo | 8/10 (80%) |
| OS | |
| Olaparib 400 mg bd | 6/10 (60%) |
| Placebo | 8/10 (80%) |
The European Medicines Agency has waived the obligation to submit the results of studies with Lynparza in all subsets of the paediatric population, in ovarian carcinoma (excluding rhabdomyosarcoma and germ cell tumours) (see section 4.2 for information on paediatric use).
The pharmacokinetics of olaparib at the 400 mg twice daily capsule dose are characterised by an apparent plasma clearance of ~8.6 L/h, an apparent volume of distribution of ~167 L and a terminal half-life of 11.9 hours.
Following oral administration of olaparib via the capsule formulation, absorption is rapid with peak plasma concentrations typically achieved between 1 to 3 hours after dosing. On multiple dosing there is no marked accumulation, with steady state exposures achieved within ~3 to 4 days.
Co-administration with food slowed the rate (tmax delayed by 2 hours) and marginally increased the extent of absorption of olaparib (AUC increased by approximately 20%). Therefore, it is recommended that patients take Lynparza at least one hour after food, and refrain from eating preferably for up to 2 hours afterwards (see section 4.2).
The in vitro protein binding is approximately 82% at clinically relevant concentrations of 10 μg/mL.
In vitro, human plasma protein binding of olaparib was dose-dependent; the fraction bound was approximately 91% at 1 μg/mL, reducing to 82% at 10 μg/mL and to 70% at 40 μg/mL. In solutions of purified proteins, the olaparib fraction bound to albumin was approximately 56%, which was independent of olaparib concentrations. Using the same assay, the fraction bound to alpha-1 acid glycoprotein was 29% at 10 μg/mL with a trend of decreased binding at higher concentrations.
In vitro, CYP3A4/5 were shown to be the enzymes primarily responsible for the metabolism of olaparib (see section 4.5).
Following oral dosing of 14C-olaparib to female patients, unchanged olaparib accounted for the majority of the circulating radioactivity in plasma (70%) and was the major component found in both urine and faeces (15% and 6% of the dose, respectively). The metabolism of olaparib is extensive. The majority of the metabolism was attributable to oxidation reactions with a number of the components produced undergoing subsequent glucuronide or sulfate conjugation. Up to 20, 37 and 20 metabolites were detected in plasma, urine and faeces respectively, the majority of them representing <1% of the dosed material. A ring-opened piperazin-3-ol moiety, and two mono-oxygenated metabolites (each ~10%) were the major circulating components, with one of the mono-oxygenated metabolites also being the major metabolite in the excreta (6% and 5% of the urinary and faecal radioactivity, respectively).
In vitro, olaparib produced little/no inhibition of UGT1A4, UGT1A9, UGT2B7, or CYPs 1A2, 2A6, 2B6, 2C8, 2C9, 2C19, 2D6 or 2E1 and is not expected to be a clinically significant time dependent inhibitor of any of these CYP enzymes. Olaparib inhibited UGT1A1 in vitro, however, PBPK simulations suggest this is not of clinical importance. In vitro, olaparib is a substrate of the efflux transporter P-gp, however this is unlikely to be of clinical significance (see section 4.5).
In vitro, data also show that olaparib is not a substrate for OATP1B1, OATP1B3, OCT1, BCRP or MRP2, and is not an inhibitor of OATP1B3, OAT1 or MRP2.
Following a single dose of 14C-olaparib, ~86% of the dosed radioactivity was recovered within a 7-day collection period, ~44% via the urine and ~42% via the faeces. Majority of the material was excreted as metabolites.
In population based PK analyses, patient age, bodyweight or race (including White and Japanese patients) were not significant covariates.
In patients with mild renal impairment (creatinine clearance 51 to 80 ml/min), AUC increased by 24% and Cmax by 15% compared with patients with normal renal function. No Lynparza dose adjustment is required for patients with mild renal impairment.
In patients with moderate renal impairment (creatinine clearance 31 to 50 ml/min), AUC increased by 44% and Cmax by 26% compared with patients with normal renal function. Lynparza dose adjustment is recommended for patients with moderate renal impairment (see section 4.2).
There are no data in patients with severe impairment or end-stage renal disease (creatinine clearance <30 ml/min).
In patients with mild hepatic impairment (Child-Pugh classification A), AUC increased by 15% and Cmax by 13% and in patients with moderate hepatic impairment (Child-Pugh classification B), AUC increased by 8% and Cmax decreased by 13% compared with patients with normal hepatic function. No Lynparza dose adjustment is required for patients with mild or moderate hepatic impairment (see section 4.2). There are no data in patients with severe hepatic impairment (Child-Pugh classification C).
No studies have been conducted to investigate the pharmacokinetics of olaparib in paediatric patients.
Olaparib showed no mutagenic potential, but was clastogenic in mammalian cells in vitro. When dosed orally to rats, olaparib induced micronuclei in bone marrow. This clastogenicity is consistent with the known pharmacology of olaparib and indicates potential for genotoxicity in man.
In repeat-dose toxicity studies of up to 6 months duration in rats and dogs, daily oral doses of olaparib were well-tolerated. The major primary target organ for toxicity in both species was the bone marrow, with associated changes in peripheral haematology parameters. These changes were reversible within 4 weeks of cessation of dosing. In rats, minimal degenerative effects on gastrointestinal tract were also noted. These findings occurred at exposures below those seen clinically. Studies using human bone marrow cells also showed that direct exposure to olaparib can result in toxicity to bone marrow cells in ex vivo assays.
In a female fertility study where rats were dosed until implantation, although extended oestrus was observed in some animals, mating performance and pregnancy rate was not affected. However, there was a slight reduction in embryofoetal survival.
In rat embryofoetal development studies, and at dose levels that did not induce significant maternal toxicity, olaparib caused reduced embryofoetal survival, reduced foetal weight and foetal developmental abnormalities, including major eye malformations (e.g. anophthalmia, microphthalmia), vertebral/rib malformation and visceral and skeletal abnormalities.
Carcinogenicity studies have not been conducted with olaparib.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.