LYUMJEV Solution for injection Ref.[10658] Active ingredients: Insulin lispro
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Eli Lilly Nederland B.V., Papendorpseweg 83, 3528 BJ Utrecht, The Netherlands
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Drugs used in diabetes, insulins and analogues for injection, fast-acting
ATC code: A10AB04
Mechanism of action
The primary activity of Lyumjev is the regulation of glucose metabolism. Insulins, including insulin lispro, the active substance in Lyumjev, exert their specific action through binding to insulin receptors. Receptor-bound insulin lowers blood glucose by stimulating peripheral glucose uptake by skeletal muscle and fat, and by inhibiting hepatic glucose production. Insulins inhibit lipolysis and proteolysis, and enhance protein synthesis.
Lyumjev is a formulation of insulin lispro that contains citrate and treprostinil. Citrate increases local vascular permeability and treprostinil induces local vasodilation to achieve accelerated absorption of insulin lispro.
Pharmacodynamic effects
Early and late insulin action
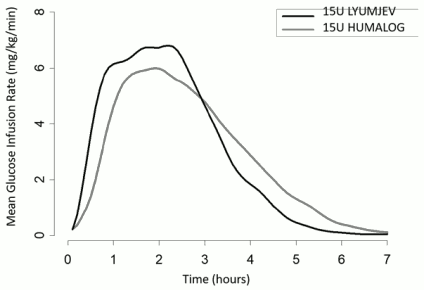
A glucose clamp study was conducted in 40 type 1 diabetes patients given Lyumjev and Humalog subcutaneously as a single 15 unit dose. Results are provided in Figure 1. Lyumjev has been shown to be equipotent to Humalog on a unit for unit basis but its effect is more rapid with a shorter duration of action.
Onset of action of Lyumjev was 20 minutes post dose, 11 minutes faster than Humalog.
During the first 30 minutes post dose, Lyumjev had a 3-fold greater glucose lowering effect compared to Humalog.
Maximum glucose-lowering effect of Lyumjev occurred between 1 and 3 hours after injection.
The late insulin action, from 4 hours until the end of the glucose clamp, was 54% lower with Lyumjev than observed with Humalog.
The duration of action of Lyumjev was 5 hours, 44 minutes shorter than Humalog.
The total glucose infused during the clamp was comparable between Lyumjev and Humalog.
Figure 1. Mean glucose infusion rate (GIR) in patients with type 1 diabetes after subcutaneous injection of Lyumjev or Humalog (15 unit dose):
Similarly, a faster early insulin action and a reduced late insulin action was observed with Lyumjev in type 2 diabetes patients.
Total and maximum glucose lowering effect of Lyumjev increased with dose within the therapeutic dose range. The early onset and total insulin action were similar when Lyumjev was administered in the abdomen, upper arm, or thigh.
Postprandial Glucose (PPG) Lowering
Lyumjev reduced the PPG during a standardized test meal over the complete 5 hour test meal period (change from premeal AUC(0-5h)) compared to Humalog.
In patients with type 1 diabetes, Lyumjev reduced the PPG during the 5 hour test meal period by 32% when given at the start of the meal and 18% when given 20 minutes after the start of the meal compared to Humalog.
In patients with type 2 diabetes, Lyumjev reduced the PPG during the 5 hour test meal period by 26% when given at the start of the meal and 24% when given 20 minutes after the start of the meal compared to Humalog.
Comparison of Lyumjev 200 units/mL and Lyumjev 100 units/mL
The maximum and total glucose lowering were comparable for Lyumjev 200 units/mL or Lyumjev 100 units/mL. No dose conversion is required if transferring a patient between the strengths.
Clinical efficacy and safety
The efficacy of Lyumjev was evaluated in 3 randomized, active controlled trials in adults.
Type 1 Diabetes – Adults
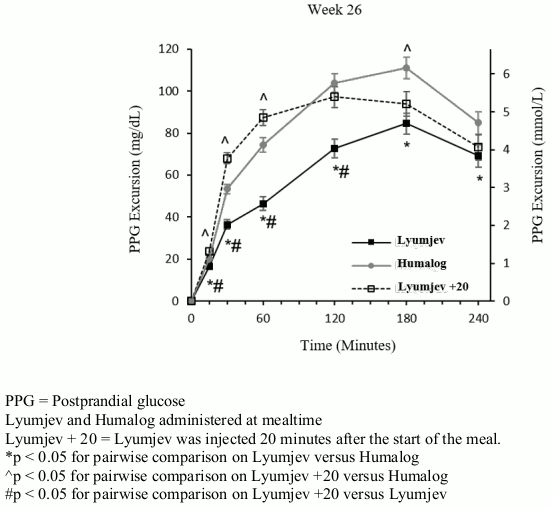
PRONTO-T1D was a 26 week, treat-to-target, trial that evaluated the efficacy of Lyumjev in 1222 patients on multiple daily injection therapy. Patients were randomized to either blinded mealtime Lyumjev, blinded mealtime Humalog, or open-label postmeal Lyumjev, all in combination with either insulin glargine or insulin degludec. Mealtime Lyumjev or Humalog was injected 0 to 2 minutes before the meal and postmeal Lyumjev was injected 20 minutes after the start of the meal.
Efficacy results are provided in Table 2 and Figure 2.
37.4% of patients treated with mealtime Lyumjev, 33.6% of patients treated with mealtime Humalog and 25.6% of patients treated with postmeal Lyumjev reached a target HbA1c of <7%.
Basal, bolus and total insulin doses were similar among study arms at 26 weeks.
Following the 26 week period, the two blinded treatment arms continued to 52 weeks. HbA1c was not statistically significantly different between treatments at the 52 week endpoint.
Table 2. Results from 26 week basal-bolus clinical trial in patients with type 1 diabetes:
| Mealtime Lyumjev + basal insulin | Mealtime Humalog + basal insulin | Postmeal Lyumjev + basal insulin | |
|---|---|---|---|
| Number of randomized subjects (N) | 451 | 442 | 329 |
| HbA1c (%) | |||
| Baseline → week 26 | 7.34 → 7.21 | 7.33 → 7.29 | 7.36 → 7.42 |
| Change from baseline | -0.13 | -0.05 | 0.08 |
| Treatment difference | -0.08 [-0.16, -0.00]C | 0.13 [0.04, 0.22]D | |
| HbA1c (mmol/mol) | |||
| Baseline → week 26 | 56.7 → 55.3 | 56.7 → 56.1 | 56.9 → 57.6 |
| Change from baseline | -1.4 | -0.6 | 0.8 |
| Treatment difference | -0.8 [-1.7, 0.00]C | 1.4 [0.5, 2.4]D | |
| 1 hour postprandial glucose excursion (mg/dL)A | |||
| Baseline → week 26 | 77.3 → 46.4 | 71.5 → 74.3 | 76.3 → 87.5 |
| Change from baseline | -28.6 | -0.7 | 12.5 |
| Treatment difference | -27.9 [-35.3, -20.6]C,E | 13.2 [5.0, 21.4]D | |
| 1 hour postprandial glucose excursion (mmol/L)A | |||
| Baseline → week 26 | 4.29 → 2.57 | 3.97 → 4.13 | 4.24 → 4.86 |
| Change from baseline | -1.59 | -0.04 | 0.70 |
| Treatment difference | -1.55 [-1.96, -1.14]C,E | 0.73 [0.28, 1.19]D | |
| 2 hour postprandial glucose excursion (mg/dL)A | |||
| Baseline → week 26 | 112.7 → 72.7 | 101.6 → 103.9 | 108.0 → 97.2 |
| Change from baseline | -34.7 | -3.5 | -10.2 |
| Treatment difference | -31.2 [-41.1, -21.2]C,E | -6.7 [-17.6, 4.3]D | |
| 2 hour postprandial glucose excursion (mmol/L)A | |||
| Baseline → week 26 | 6.26 → 4.04 | 5.64 → 5.77 | 5.99 → 5.40 |
| Change from baseline | -1.93 | -0.20 | -0.56 |
| Treatment difference | -1.73 [-2.28, -1.18]C,E | -0.37 [-0.98, -0.24]D | |
| Body weight (Kg) | |||
| Baseline → week 26 | 77.3 → 77.9 | 77.3 → 78.2 | 77.6 → 78.1 |
| Change from baseline | 0.6 | 0.8 | 0.7 |
| Treatment difference | -0.2 [-0.6, 0.1]A | -0.1[-0.5, 0.3]D | |
| Severe hypoglycaemiaB (% of patients) | 5.5% | 5.7% | 4.6% |
Week 26 and change from baseline values are based on the least-squares means (adjusted means).
The 95% confidence interval is stated in '[ ]'.
A Meal test
B Severe hypoglycaemia is defined as episode requiring assistance of another person due to patient's neurological impairment.
C The difference is for mealtime Lyumjev – mealtime Humalog.
D The difference is for postmeal Lyumjev – mealtime Humalog.
E Statistically significant in favour of mealtime Lyumjev.
Figure 2. Time course of blood glucose excursion during mixed-meal tolerance test at week 26 in patients with type 1 diabetes:
Continuous glucose monitoring (CGM) in Type 1 Diabetes – Adults
A subset of patients (N=269) participated in an evaluation of the 24 hour ambulatory glucose profiles captured with blinded CGM. At the 26 week assessment, patients treated with mealtime Lyumjev demonstrated statistically significant improvement in PPG control during CGM assessment of glucose excursions or incremental area under the curve (AUC) 0-2 hours, 0-3 hours, and 0-4 hours after meals compared to patients treated with Humalog. Patients treated with mealtime Lyumjev reported statistically significantly longer time in range (6 am to midnight) with 603 minutes in range, (3.9 to 10 mmol/L, 71-180 mg/dL), and 396 minutes in range (3.9 to 7.8 mmol/L, 71 to 140 mg/dL), 44 and 41 minutes longer than Humalog patients respectively.
Type 2 Diabetes – Adults
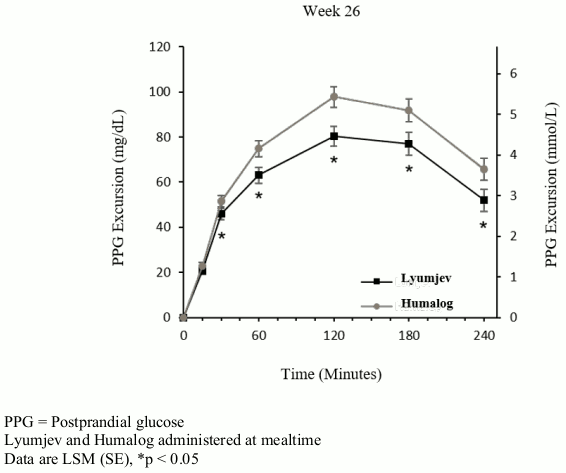
PRONTO-T2D was a 26 week, treat-to-target trial that evaluated the efficacy of Lyumjev in 673 patients were randomized to either blinded mealtime Lyumjev or to blinded mealtime Humalog, both in combination with a basal insulin (insulin glargine or insulin degludec) in a basal-bolus regimen. Mealtime Lyumjev or mealtime Humalog was injected 0-2 minutes before the meal. Efficacy results are provided in Table 3 and Figure 3.
58.2% of patients treated with mealtime Lyumjev and 52.5% of patients treated with mealtime Humalog reached a target HbA1c of <7%.
Basal, bolus and total insulin doses were similar among study arms at the end of the trial.
Table 3. Results from 26 week basal-bolus clinical trial in patients with type 2 diabetes:
| Mealtime Lyumjev + basal insulin | Mealtime Humalog + basal insulin | |
|---|---|---|
| Number of randomized subjects (N) | 336 | 337 |
| HbA1c (%) | ||
| Baseline → week 26 | 7.28 → 6.92 | 7.31 → 6.86 |
| Change from baseline | -0.38 | -0.43 |
| Treatment difference | 0.06 [-0.05, 0.16] | |
| HbA1c (mmol/mol) | ||
| Baseline → week 26 | 56.0 → 52.1 | 56.4 → 51.5 |
| Change from baseline | -4.1 | -4.7 |
| Treatment difference | 0.6 [-0.6, 1.8] | |
| 1 hour postprandial glucose excursion (mg/dL)A | ||
| Baseline → week 26 | 76.6 → 63.1 | 77.1 → 74.9 |
| Change from baseline | -13.8 | -2.0 |
| Treatment difference | -11.8 [-18.1, -5.5]C | |
| 1 hour postprandial glucose excursion (mmol/L)A | ||
| Baseline → week 26 | 4.25 → 3.50 | 4.28 → 4.16 |
| Change from baseline | -0.77 | -0.11 |
| Treatment difference | -0.66 [-1.01, -0.30]C | |
| 2 hour postprandial glucose excursion (mg/dL)A | ||
| Baseline → week 26 | 99.3 → 80.4 | 99.6 → 97.8 |
| Change from baseline | -19.0 | -1.6 |
| Treatment difference | -17.4 [-25.3, -9.5]C | |
| 2 hour postprandial glucose excursion (mmol/L)A | ||
| Baseline → week 26 | 5.51 → 4.47 | 5.53 → 5.43 |
| Change from baseline | -1.06 | -0.09 |
| Treatment difference | -0.96 [-1.41, -0.52]C | |
| Body weight (Kg) | ||
| Baseline → week 26 | 89.8 → 91.3 | 90.0 → 91.6 |
| Change from baseline | 1.4 | 1.7 |
| Treatment difference | -0.2 [-0.7, 0.3] | |
| Severe hypoglycaemia (% of patients)B | 0.9% | 1.8% |
Week 26 and change from baseline values are based on the least-squares means (adjusted means).
The 95% confidence interval is stated in '[ ]'. The difference is for mealtime Lyumjev – mealtime Humalog.
A Meal test
B Severe hypoglycaemia is defined as episode requiring assistance of another person due to patient's neurological impairment.
C Statistically significant in favour of mealtime Lyumjev.
Figure 3. Time course of blood glucose excursion during mixed-meal tolerance test at week 26 in patients with type 2 diabetes:
Elderly
In the two 26 week clinical studies, 187 of 1,116 (17%) Lyumjev treated patients with type 1 diabetes or type 2 diabetes were ≥65 years of age and 18 of 1,116 (2%) were ≥75 years of age. No overall differences in safety or effectiveness were observed between elderly patients and younger patients.
Type 1 Diabetes – Adults. CSII
PRONTO-Pump was a 12 week cross over design (2 periods of 6 weeks), double-blind, trial that evaluated the compatibility and safety of Lyumjev and Humalog with an external CSII System in patients who wore a continuous glucose monitor throughout the study. There were no statistically significant treatment difference in the rate or incidence of infusion set failures (n=49).
In period 1 of the cross over study, Lyumjev had a numerically greater reduction in mean HbA1c than Humalog. Lyumjev reduction was -0.39% [-4.23 mmol/mol] from a baseline of 6.97% [52.68 mmol/mol] and Humalog reduction was -0.25% [-2.78 mmol/mol] from a baseline of 7.17% [54.89 mmol/mol]. Lyumjev had a statistically significantly longer mean duration of time with glucose in target ranges 71-140 mg/dL (3.9 to 7.8 mmol/L) within 1 and 2 hours after the start of breakfast compared to Humalog.
5.2. Pharmacokinetic properties
Absorption
Absorption of insulin lispro was accelerated and the duration of exposure was shorter in healthy subjects and patients with diabetes following injection of Lyumjev compared to Humalog. In patients with type 1 diabetes:
- Insulin lispro appeared in circulation approximately 1 minute after injection of Lyumjev, which was five minutes faster than Humalog.
- Time to 50% maximum concentration was 14 minutes shorter with Lyumjev compared to Humalog.
- Following injection of Lyumjev, there was seven times more insulin lispro in circulation during the first 15 minutes compared to Humalog and three times more insulin lispro during the first 30 minutes compared to Humalog.
- After administration of Lyumjev the time to maximum insulin lispro concentration was achieved at 57 minutes.
- Following injection of Lyumjev there was 41% less insulin lispro in circulation after 3 hours following injection compared to Humalog.
- The duration of insulin lispro exposure for Lyumjev was 60 minutes shorter compared to Humalog.
- The total insulin lispro exposure (ratio and 95% CI of 1.03 (0.973, 1.09) and maximum concentration (ratio and 95% CI of 1.06 (0.97, 1.16) were comparable between Lyumjev and Humalog.
In type 1 patients, the day-to-day variability [CV%] of Lyumjev was 13% for total insulin lispro exposure (AUC, 0-10h) and 23% for maximum insulin lispro concentration (Cmax).The absolute bioavailability of insulin lispro after subcutaneous administration of Lyumjev in the abdomen, upper arm and thigh was approximately 65%. The accelerated absorption of insulin lispro is maintained regardless of injection site (abdomen, upper arm and thigh). No exposure data are available following injection in the buttocks.
Maximum concentration and time to maximum concentration were comparable for the abdomen and upper arm regions; time to maximum concentration was longer and maximum concentration lower for the thigh.
Total insulin lispro exposure and maximum insulin lispro concentration increased proportionally with increasing subcutaneous doses of Lyumjev within the dose range from 7U to 30U.
CSII
The absorption of insulin lispro was accelerated when Lyumjev was administered by CSII in patients with type 1 diabetes.
- Time to reach 50% maximum concentration was 14 minutes, 9 minutes shorter than for Humalog.
- Following administration of Lyumjev, 1.5 times more insulin lispro was available during the first 30 minutes compared to Humalog.
Comparison of Lyumjev 200 units/mL and Lyumjev 100 units/mL
The results of a study in healthy subjects demonstrated that Lyumjev 200 units/mL is bioequivalent to Lyumjev 100 units/mL following administration of a single 15 unit dose for the area under serum insulin lispro concentration-time curve from time zero to infinity and maximum insulin lispro concentration. The accelerated insulin lispro absorption after administration of 200 units/mL was similar to that observed with Lyumjev 100 units/mL. No dose conversion is required if transferring a patient between the strengths.
Distribution
The geometric mean (% coefficient of variation [CV%]) volume of distribution of insulin lispro (Vd) was 34 L (30%) after intravenous administration of Lyumjev as a bolus injection of a 15 unit dose in healthy subjects.
Elimination
The geometric mean (CV%) clearance of insulin lispro was 32 L/hour (22%) and the median half-life of insulin lispro was 44 minutes after intravenous administration of Lyumjev as a bolus injection of a 15 unit dose in healthy subjects.
Special populations
In adult subjects, age, gender, and race did not affect the pharmacokinetics and pharmacodynamics of Lyumjev. No data are available on children and adolescents below 18 years of age.
Patients with renal and hepatic impairment
Renal and hepatic impairment is not known to impact the pharmacokinetics of insulin lispro.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity, carcinogenic potential, toxicity to reproduction and development after exposure to insulin lispro.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.