MAVIRET Film-coated tablet Ref.[10436] Active ingredients: Glecaprevir Glecaprevir and Pibrentasvir Pibrentasvir
Source: European Medicines Agency (EU) Revision Year: 2023 Publisher: bbVie Deutschland GmbH & Co. KG, Knollstrasse, 67061 Ludwigshafen, Germany
4.3. Contraindications
Hypersensitivity to the active substances or to any of the excipients listed in section 6.1.
Patients with severe hepatic impairment (Child-Pugh C) (see sections 4.2, 4.4, and 5.2).
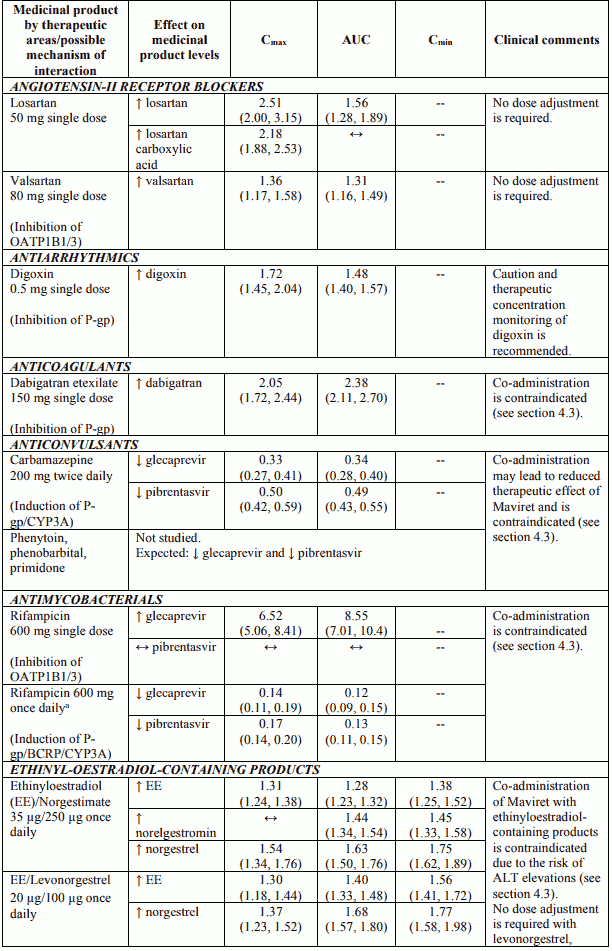
Concomitant use with atazanavir containing products, atorvastatin, simvastatin, dabigatran etexilate, ethinyl oestradiol-containing products, strong P-gp and CYP3A inducers (e.g., rifampicin, carbamazepine, St. John’s wort (Hypericum perforatum), phenobarbital, phenytoin, and primidone) (see section 4.5).
4.4. Special warnings and precautions for use
Hepatitis B Virus reactivation
Cases of hepatitis B virus (HBV) reactivation, some of them fatal, have been reported during or after treatment with direct acting antiviral agents. HBV screening should be performed in all patients before initiation of treatment. HBV/HCV co-infected patients are at risk of HBV reactivation, and should, therefore, be monitored and managed according to current clinical guidelines.
Hepatic impairment
Maviret is not recommended in patients with moderate hepatic impairment (Child-Pugh B) and is contraindicated in patients with severe hepatic impairment (Child-Pugh C) (see sections 4.2, 4.3, and 5.2).
Patients who failed a prior regimen containing an NS5A- and/or an NS3/4A-inhibitor
Genotype 1-infected (and a very limited number of genotype 4-infected) patients with prior failure on regimens that may confer resistance to glecaprevir/pibrentasvir were studied in studies MAGELLAN-1 and B16-439 (section 5.1). The risk of failure was, as expected, highest for those exposed to both classes. A resistance algorithm predictive of the risk for failure by baseline resistance has not been established. Accumulating double class resistance was a general finding for patients who failed re-treatment with glecaprevir/pibrentasvir in MAGELLAN-1.
No re-treatment data is available for patients infected with genotypes 2, 3, 5 or 6. Maviret is not recommended for the re-treatment of patients with prior exposure to NS3/4A- and/or NS5A inhibitors.
Drug-drug interactions
Co-administration is not recommended with several medicinal products as detailed in section 4.5.
Use in diabetic patients
Diabetics may experience improved glucose control, potentially resulting in symptomatic hypoglycaemia, after initiating HCV direct acting antiviral treatment. Glucose levels of diabetic patients initiating direct acting antiviral therapy should be closely monitored, particularly within the first 3 months, and their diabetic medicines modified when necessary. The physician in charge of the diabetic care of the patient should be informed when direct acting antiviral therapy is initiated.
Lactose
Maviret contains lactose. Patients with rare hereditary problems of galactose intolerance, total lactase deficiency or glucose-galactose malabsorption should not take this medicinal product.
Sodium
This medicinal product contains less than 1 mmol sodium (23 mg) per tablet, that is to say essentially ‘sodium-free’.
4.5. Interaction with other medicinal products and other forms of interaction
Potential for Maviret to affect other medicinal products
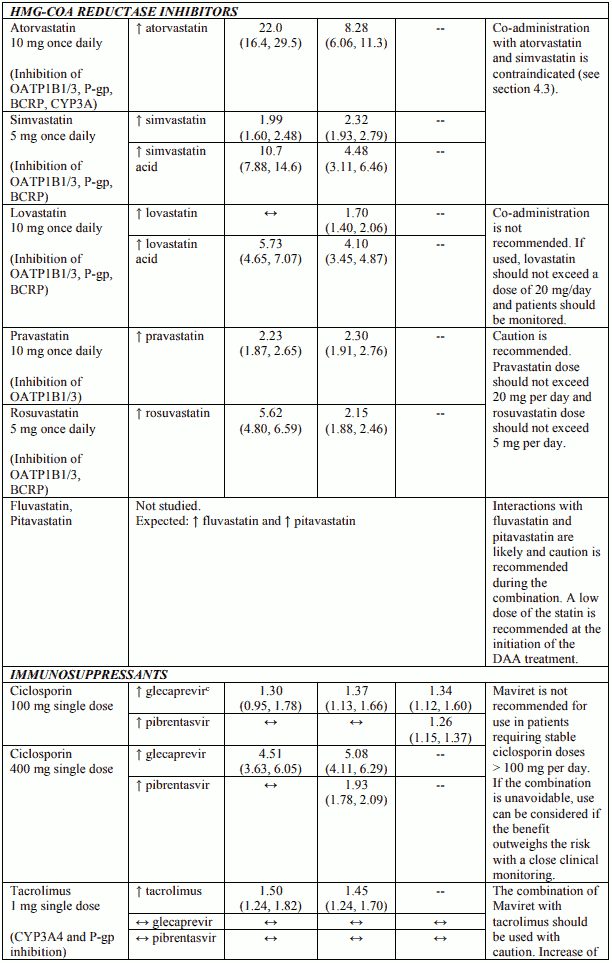
Glecaprevir and pibrentasvir are inhibitors of P-glycoprotein (P-gp), breast cancer resistance protein (BCRP), and organic anion transporting polypeptide (OATP) 1B1/3. Co-administration with Maviret may increase plasma concentrations of medicinal products that are substrates of P-gp (e.g. dabigatran etexilate, digoxin), BCRP (e.g. rosuvastatin), or OATP1B1/3 (e.g. atorvastatin, lovastatin, pravastatin, rosuvastatin, simvastatin). See Table 3 for specific recommendations on interactions with sensitive substrates of P-gp, BCRP, and OATP1B1/3. For other P-gp, BCRP, or OATP1B1/3 substrates, dose adjustment may be needed.
Glecaprevir and pibrentasvir are weak inhibitors of cytochrome P450 (CYP) 3A and uridine glucuronosyltransferase (UGT) 1A1 in vivo. Clinically significant increases in exposure were not observed for sensitive substrates of CYP3A (midazolam, felodipine) or UGT1A1 (raltegravir) when administered with Maviret.
Both glecaprevir and pibrentasvir inhibit the bile salt export pump (BSEP) in vitro.
Significant inhibition of CYP1A2, CYP2C9, CYP2C19, CYP2D6, UGT1A6, UGT1A9, UGT1A4, UGT2B7, OCT1, OCT2, OAT1, OAT3, MATE1 or MATE2K are not expected.
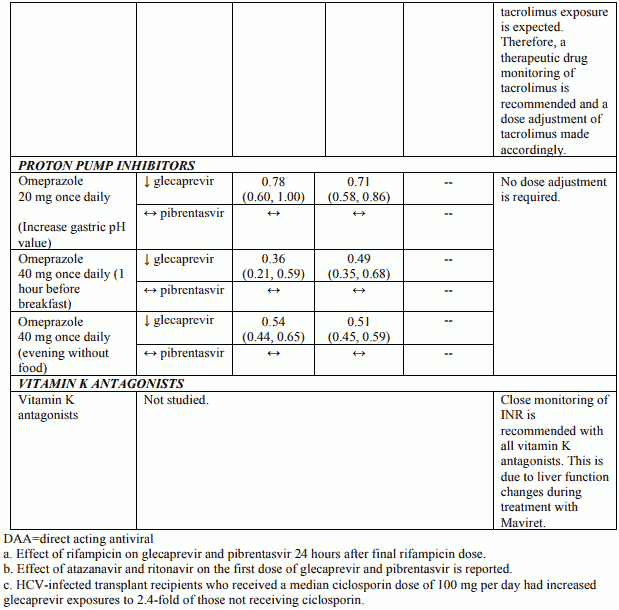
Patients treated with vitamin K antagonists
As liver function may change during treatment with Maviret, a close monitoring of International Normalised Ratio (INR) values is recommended.
Potential for other medicinal products to affect Maviret
Use with strong P-gp/CYP3A inducers
Medicinal products that are strong P-gp and CYP3A inducers (e.g., rifampicin, carbamazepine, St. John’s wort (Hypericum perforatum), phenobarbital, phenytoin, and primidone) could significantly decrease glecaprevir or pibrentasvir plasma concentrations and may lead to reduced therapeutic effect of Maviret or loss of virologic response. Co-administration of such medicinal products with Maviret is contraindicated (see section 4.3).
Co-administration of Maviret with medicinal products that are moderate inducers P-gp/CYP3A may decrease glecaprevir and pibrentasvir plasma concentrations (e.g. oxcarbazepine, eslicarbazepine, lumacaftor, crizotinib). Co-administration of moderate inducers is not recommended (see section 4.4).
Glecaprevir and pibrentasvir are substrates of the efflux transporters P-gp and/or BCRP. Glecaprevir is also a substrate of the hepatic uptake transporters OATP1B1/3. Co-administration of Maviret with medicinal products that inhibit P-gp and BCRP (e.g. ciclosporin, cobicistat, dronedarone, itraconazole, ketoconazole, ritonavir) may slow elimination of glecaprevir and pibrentasvir and thereby increase plasma exposure of the antivirals. Medicinal products that inhibit OATP1B1/3 (e.g. elvitegravir, ciclosporin, darunavir, lopinavir) increase systemic concentrations of glecaprevir.
Established and other potential medicinal product interactions
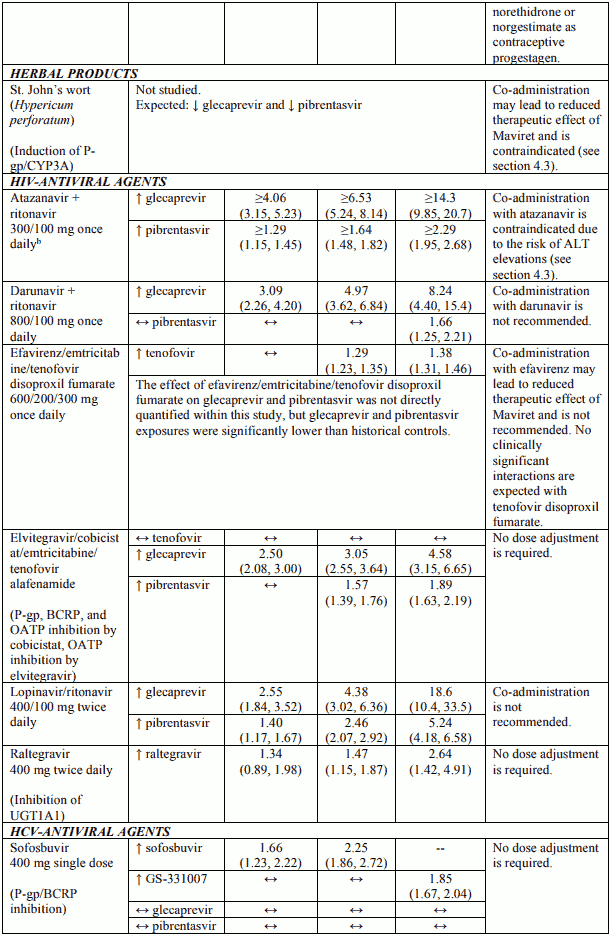
Table 3 provides the least-squares mean Ratio (90% Confidence Interval) effect on concentration of Maviret and some common concomitant medicinal products. The direction of the arrow indicates the direction of the change in exposures (Cmax, AUC, and Cmin) in glecaprevir, pibrentasvir, and the coadministered medicinal product (↑ = increase (more than 25%), ↓ = decrease (more than 20%), ↔ = no change (equal to or less than 20% decrease or 25% increase)). This is not an exclusive list.
Table 3. Interactions between Maviret and other medicinal products:
Additional drug-drug interaction studies were performed with the following medical products and showed no clinically significant interactions with Maviret: abacavir, amlodipine, buprenorphine, caffeine, dextromethorphan, dolutegravir, emtricitabine, felodipine, lamivudine, lamotrigine, methadone, midazolam, naloxone, norethindrone or other progestin-only contraceptives, rilpivirine, tenofovir alafenamide and tolbutamide.
4.6. Fertility, pregnancy and lactation
Pregnancy
There are no or limited amount of data (less than 300 pregnancy outcomes) from the use of glecaprevir or pibrentasvir in pregnant women.
Studies in rats/mice with glecaprevir or pibrentasvir do not indicate direct or indirect harmful effects with respect to reproductive toxicity. Maternal toxicity associated with embryo-foetal loss has been observed in the rabbit with glecaprevir which precluded evaluation of glecaprevir at clinical exposures in this species (see section 5.3). As a precautionary measure, Maviret use is not recommended in pregnancy.
Breast-feeding
It is unknown whether glecaprevir or pibrentasvir are excreted in human milk. Available pharmacokinetic data in animals have shown excretion of glecaprevir and pibrentasvir in milk (for details see section 5.3). A risk to the suckling child cannot be excluded. A decision must be made whether to discontinue breast-feeding or to discontinue/abstain from Maviret therapy taking into account the benefit of breast-feeding for the child and the benefit of therapy for the woman.
Fertility
No human data on the effect of glecaprevir and/or pibrentasvir on fertility are available. Animal studies do not indicate harmful effects of glecaprevir or pibrentasvir on fertility at exposures higher than the exposures in humans at the recommended dose (see Section 5.3).
4.7. Effects on ability to drive and use machines
Maviret has no or negligible influence on the ability to drive and use machines.
4.8. Undesirable effects
Summary of the safety profile
In pooled Phase 2 and 3 clinical studies of adult subjects receiving Maviret with genotype 1, 2, 3, 4, 5 or 6 HCV infection the most commonly reported adverse reactions (incidence ≥ 10%) were headache and fatigue. Less than 0.1% of subjects treated with Maviret had serious adverse reactions (transient ischaemic attack). The proportion of subjects treated with Maviret who permanently discontinued treatment due to adverse reactions was 0.1%.
Tabulated list of adverse reactions
The following adverse reactions were identified in registrational Phase 2 and 3 studies in HCVinfected adults with or without cirrhosis treated with Maviret for 8, 12 or 16 weeks, or during postmarketing experience. The adverse reactions are listed below by body system organ class and frequency. Frequencies are defined as follows: very common (≥1/10), common (≥1/100 to <1/10), uncommon (≥1/1 000 to <1/100), rare (≥1/10 000 to <1/1 000), very rare (<1/10 000) or not known (cannot be estimated from the available data).
Table 4. Adverse reactions identified with Maviret:
| Frequency | Adverse reactions |
|---|---|
| Immune system disorders | |
| Uncommon | angioedema |
| Nervous system disorders | |
| Very common | headache |
| Gastrointestinal disorders | |
| Common | diarrhoea, nausea |
| Skin and subcutaneous tissue disorders | |
| Not known | pruritus |
| General disorders and administration site conditions | |
| Very common | fatigue |
| Common | asthenia |
| Investigations | |
| Common | elevation in total bilirubin |
Description of selected adverse reactions
Adverse reactions in subjects with severe renal impairment including subjects on dialysis
The safety of Maviret in subjects with chronic kidney disease (including subjects on dialysis) and genotypes 1, 2, 3, 4, 5 or 6 chronic HCV infection with compensated liver disease (with or without cirrhosis) was assessed in adults in EXPEDITION-4 (n=104) and EXPEDITION-5 (n=101). The most common adverse reactions in subjects with severe renal impairment were pruritus (17%) and fatigue (12%) in EXPEDITION-4 and pruritus (14.9%) in EXPEDITION-5.
Adverse reactions in subjects with liver or kidney transplant
The safety of Maviret was assessed in 100 post-liver or -kidney transplant adult recipients with genotypes 1, 2, 3, 4, or 6 chronic HCV infection without cirrhosis (MAGELLAN-2). The overall safety profile in transplant recipients was comparable to that observed in subjects in the Phase 2 and 3 studies. Adverse reactions observed in greater than or equal to 5% of subjects receiving Maviret for 12 weeks were headache (17%), fatigue (16%), nausea (8%) and pruritus (7%).
Safety in HCV/HIV-1 co-infected subjects
The overall safety profile in HCV/HIV-1 co-infected adult subjects (ENDURANCE-1 and EXPEDITION-2) was comparable to that observed in HCV mono-infected adult subjects.
Paediatric population
The safety of Maviret in HCV GT1-6 infected adolescents is based on data from a Phase ⅔ open-label study in 47 subjects aged 12 years to < 18 years treated with Maviret for 8 to 16 weeks (DORA Part 1). The adverse reactions observed were comparable with those observed in clinical studies of Maviret in adults.
Serum bilirubin elevations
Elevations in total bilirubin of at least 2x upper limit normal (ULN) were observed in 1.3% of subjects related to glecaprevir-mediated inhibition of bilirubin transporters and metabolism. Bilirubin elevations were asymptomatic, transient, and typically occurred early during treatment. Bilirubin elevations were predominantly indirect and not associated with ALT elevations. Direct hyperbilirubinemia was reported in 0.3% of subjects.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system listed in Appendix V.
6.2. Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.