MENOSTAR Patch Ref.[50375] Active ingredients: Estradiol
Source: FDA, National Drug Code (US) Revision Year: 2021
Product description
Menostar (estradiol transdermal system) is designed to provide nominal in vivo delivery of 14 mcg of estradiol per day continuously upon application to intact skin. The period of use is 7 days. The transdermal system has a contact surface area of 3.25 cm², and contains 1 mg of estradiol USP.
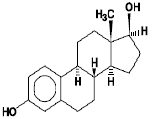
Estradiol USP is a white, crystalline powder, chemically described as estra-1,3,5(10)-triene-3, 17ß-diol. It has an empirical formula of C18H24O2 and molecular weight of 272.38. The structural formula is:
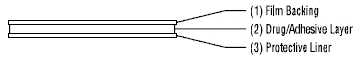
The Menostar transdermal system comprises three layers. Proceeding from the visible surface toward the surface attached to the skin, these layers are:
1. A translucent polyethylene film.
2. An acrylate adhesive matrix containing estradiol USP.
3. A protective liner of siliconized or fluoropolymer-coated polyester film is attached to the adhesive surface and must be removed before the transdermal system can be used.
The active component of the transdermal system is estradiol. The remaining components of the transdermal system (acrylate copolymer adhesive, fatty acid esters, and polyethylene backing) are pharmacologically inactive.
| Dosage Forms and Strengths |
|---|
|
Menostar (estradiol transdermal system) 14 mcg per day - each 3.25 cm² system contains 1 mg of estradiol. |
| How Supplied |
|---|
|
Menostar (estradiol transdermal system), 14 mcg per day — each 3.25 cm² system contains 1 mg of estradiol USP. Individual Carton of 4 systems NDC 50419-455-04. Manufactured for Bayer HealthCare Pharmaceuticals Inc., Whippany, NJ 07981 |
Drugs
| Drug | Countries | |
|---|---|---|
| MENOSTAR | United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.