MEPROBAMATE Tablet Ref.[51056] Active ingredients: Meprobamate
Source: FDA, National Drug Code (US) Revision Year: 2023
Product description
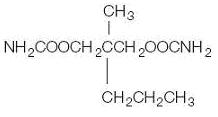
Meprobamate is a white powder with a characteristic odor and a bitter taste. It is slightly soluble in water, freely soluble in acetone and alcohol, and sparingly soluble in ether. The structural formula of meprobamate is:
C9H18N2O4 M.W. 218.25
Meprobamate Tablets USP 200 mg and 400 mg for oral administration contain the following inactive ingredients: colloidal silicon dioxide, magnesium stearate, microcrystalline cellulose, sodium starch glycolate and pregelatinised starch.
| How Supplied |
|---|
|
Meprobamate Tablets USP 200 mg are white to off white, round, biconvex, uncoated tablets debossed with “L125” on one side and break line on other side. NDC 46708-019-30 Bottle of 30 Meprobamate Tablets USP 400 mg are white to off white, round, biconvex, uncoated tablets debossed with “L105” on one side and break line on other side. NDC 46708-020-30 Bottle of 30 Manufactured by: Alembic Pharmaceuticals Limited (Formulation Division), Village Panelav, P. O. Tajpura, Near Baska, Taluka-Halol, Panchmahal, Gujarat, India |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.