MIACALCIN Solution for injection Ref.[10594] Active ingredients: Calcitonin
Source: FDA, National Drug Code (US) Revision Year: 2018
Product description
Calcitonin is a polypeptide hormone secreted by the parafollicular cells of the thyroid gland in mammals and by the ultimobranchial gland of birds and fish.
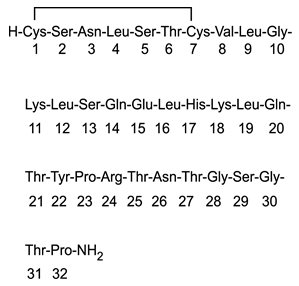
Miacalcin (calcitonin salmon, USP) Injection, Synthetic is a synthetic polypeptide of 32 amino acids in the same linear sequence that is found in calcitonin of salmon origin. This is shown by the following graphic formula:
It is provided in sterile solution for subcutaneous or intramuscular injection. Each milliliter contains: calcitonin salmon, 200 USP Units.
Inactive Ingredients (per mL): acetic acid, USP, 2.25 mg; phenol, USP, 5.0 mg; sodium acetate trihydrate, USP, 2.0 mg; sodium chloride, USP, 7.5 mg; water for injection, USP.
The activity of Miacalcin injection is stated in International Units based on bioassay in comparison with the International Reference Preparation of calcitonin salmon for Bioassay, distributed by the National Institute for Biological Standards and Control, Holly Hill, London.
| Dosage Forms and Strengths |
|---|
|
Miacalcin injection is available as a clear, colorless, sterile solution of synthetic calcitonin salmon, USP in individual 2 mL multi-dose vials containing 200 USP Units per mL. |
| How Supplied |
|---|
|
Miacalcin (calcitonin salmon, USP) Injection, Synthetic is available as a sterile solution in individual 2 mL multi-dose vials containing 200 USP Units per mL. NDC 67457-675-02 - carton containing 1 x 2 mL multi-dose vial Manufactured for: Mylan Institutional LLC, Rockford, IL 61103 U.S.A. Manufactured by: Alcami Corporation, Charleston, SC 29405 U.S.A. |
Drugs
| Drug | Countries | |
|---|---|---|
| MIACALCIN | United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.