MIEBO Ophthalmic solution Ref.[107374] Active ingredients: Perfluorohexyloctane
Source: FDA, National Drug Code (US) Revision Year: 2023
1. Indications and Usage
MIEBO (perfluorohexyloctane ophthalmic solution) is indicated for the treatment of the signs and symptoms of dry eye disease (DED).
2. Dosage and Administration
2.1 Recommended Dosage
Instill one drop of MIEBO four times daily into affected eye(s).
Contact lenses should be removed prior to and for at least 30 minutes after the administration of MIEBO.
2.2 Administration Instructions
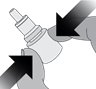
Step 1. Remove the cap from eye drop bottle.
Step 2. Holding the bottle upright, gently squeeze the bottle.
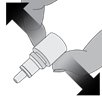
Step 3. While squeezing, turn the bottle upside down and release the pressure (drawing air into the bottle).
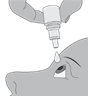
Step 4. Keeping the bottle upside down, place the bottle above your eye and squeeze it again to release a drop into your eye. Repeat steps 1–4 for the second affected eye.
16.2. Storage and Handling
Store MIEBO at 15ºC to 25ºC (59ºF to 77ºF). After opening, MIEBO can be used until the expiration date on the bottle.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.