MINIDIAB Tablet Ref.[50309] Active ingredients: Glipizide
Source: Pharmaceutical Benefits Scheme (AU) Revision Year: 2020 Publisher: Pfizer Australia Pty Ltd, Level 17, 151 Clarence Street, Sydney NSW 2000, Toll Free Number: 1800 675 229, www.pfizer.com.au
Product name and form
MINIDIAB - Glipizide.
| Pharmaceutical Form |
|---|
|
White, round, biconvex, scored tablets. |
Qualitative and quantitative composition
Each tablet contains 5 mg of glipizide.
Excipient(s) with known effect: lactose monohydrate.
For the full list of excipients, see Section 6.1 List of excipients.
Physicochemical properties
Glipizide is a whitish, odourless powder with a pKa of 5.9. It is insoluble in water and alcohols, but soluble in 0.1 N NaOH; it is freely soluble in dimethylformamide.
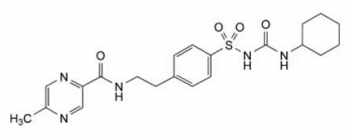
MINIDIAB (glipizide) is an oral blood-glucose lowering drug of the sulphonylurea class. The chemical name of glipizide is 1-cyclohexyl-3-{4-[2-(5-methylpyrazine-2-carboxamido)ethyl]-benzene-sulphonyl} urea. The molecular formula is C21H27N5O4S, molecular weight is 445.5, and the structural formula is shown below:
Chemical structure
CAS number: 29094-61-9
| Active Ingredient |
|---|
|
Glipizide is an oral blood-glucose-lowering drug of the sulfonylurea class. The primary mode of action of glipizide is the stimulation of insulin secretion from the beta-cells of pancreatic islet tissue. |
| List of Excipients |
|---|
|
Cellulose |
Pack sizes and marketing
The tablets are blister packed and available in pack size of 100 tablets.
Marketing authorization holder
Pfizer Australia Pty Ltd, Level 17, 151 Clarence Street, Sydney NSW 2000, Toll Free Number: 1800 675 229, www.pfizer.com.au
Marketing authorization dates and numbers
23 September 1991
Drugs
| Drug | Countries | |
|---|---|---|
| MINIDIAB | Austria, Australia, Brazil, Hong Kong, New Zealand, Singapore, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.