MIOSTAT Solution for injection Ref.[10040] Active ingredients: Carbachol
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
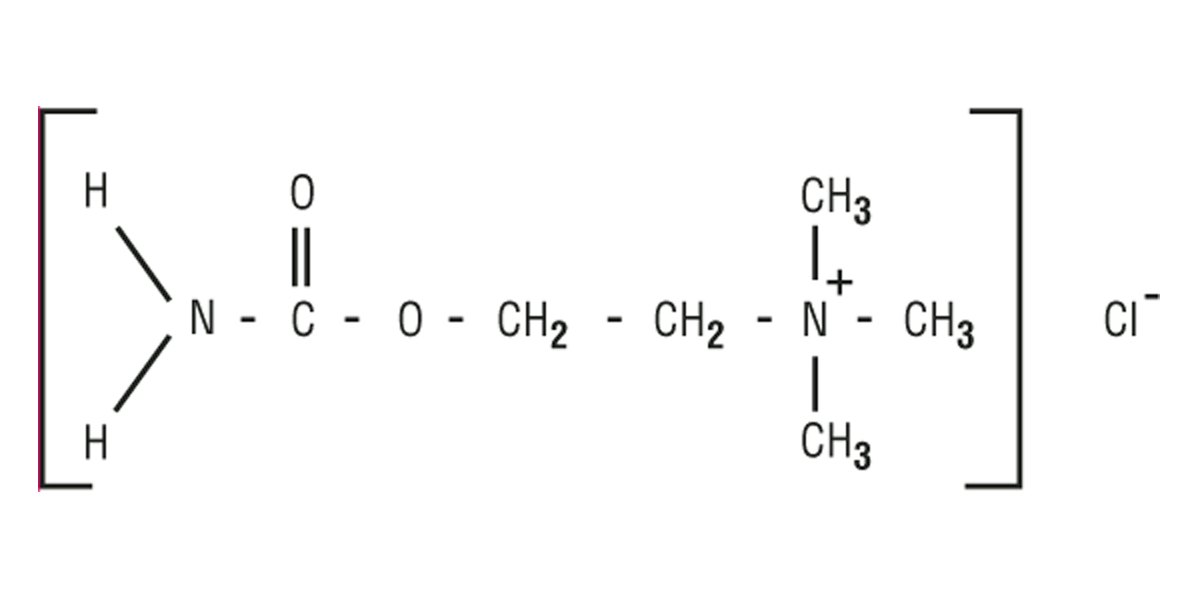
MIOSTAT (carbachol intraocular solution, USP) 0.01% is a sterile balanced salt solution of carbachol for intraocular injection. The active ingredient is represented by the chemical structure:
Established Name: Carbachol
Chemical Name: Ethanaminium, 2-[(aminocarbonyl)oxy]N,N,Ntrimethyl, chloride.
Molecular Formula: C6H15CIN2O2
Molecular Weight: 182.65
Each mL of MIOSTAT (carbachol intraocular solution, USP) 0.01%contains: Active: carbachol 0.01%.
Inactives: sodium chloride 0.64%, potassium chloride 0.075%, calcium chloride dehydrate 0.048%, magnesium chloride hexahydrate 0.03%, sodium acetate trihydrate 0.39%, sodium citrate dihydrate 0.17%, sodium hydroxide and/or hydrochloric acid (to adjust pH) and Water for Injection. pH range is 6.5-7.5.
| How Supplied |
|---|
|
In a 2.0 mL glass vial with a 1.5 mL fill, grey butyl stopper and aluminum seal packaged twelve to a carton. NDC 0065-0023-15 Distributed by: Alcon Laboratories, Inc., Fort Worth, Texas 76134 |
Drugs
| Drug | Countries | |
|---|---|---|
| MIOSTAT | Canada, Ecuador, Estonia, France, Hong Kong, Netherlands, New Zealand, Poland, Singapore, Turkey, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.