MIVACRON Liquid for injection Ref.[9356] Active ingredients:
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2017 Publisher: Aspen Pharma Trading Limited, 3016 Lake Drive, City West Business Campus, Dublin 24, Ireland, Service-Tel: 0800 008 7392 (+44 1748 828 391)
Therapeutic indications
Mivacron is a highly selective, short-acting, non-depolarising neuromuscular blocking agent with a fast recovery profile. Mivacron is used as an adjunct to general anaesthesia to relax skeletal muscles and to facilitate tracheal intubation and mechanical ventilation in adults, children and infants 2 months and over.
This formulation contains no antimicrobial preservative and is intended for single patient use.
Posology and method of administration
Use by Injection In Adults
Mivacron is administered by intravenous injection. The mean dose required to produce 95% suppression of the adductor pollicis single twitch response to ulnar nerve stimulations (ED95) is 0.07 mg/kg (range 0.06 to 0.09) in adults receiving narcotic anaesthesia.
The recommended bolus dose range for healthy adults is 0.07-0.25 mg/kg. The duration of neuromuscular blockade is related to the dose. Doses of 0.07, 0.15, 0.20 and 0.25 mg/kg produce clinically effective block for approximately 13, 16, 20 and 23 minutes respectively.
Doses of up to 0.15 mg/kg may be administered over 5 to 15 seconds. Higher doses should be administered over 30 seconds in order to minimise the possibility of occurrence of cardiovascular effects.
The following dose regimens are recommended for tracheal intubation:
I A dose of 0.2 mg/kg, administered over 30 seconds, produces good to excellent conditions for tracheal intubation within 2 to 2.5 minutes.
II A dose of 0.25 mg/kg administered as a divided dose (0.15 mg/kg followed 30 seconds later by 0.1 mg/kg) produces good to excellent conditions for tracheal intubation within 1.5 to 2.0 minutes of completion of administration of the first dose portion.
With Mivacron, significant train-of-four fade is not seen during onset. It is often possible to intubate the trachea before complete abolition of the train-of-four response of the adductor pollicis muscle has occurred.
Full block can be prolonged by maintenance doses of Mivacron. Doses of 0.1 mg/kg administered during narcotic anaesthesia each provide approximately 15 minutes of additional clinically effective block. Successive supplementary doses do not give rise to accumulation of neuromuscular blocking effect.
The neuromuscular blocking action of Mivacron is potentiated by isoflurane or enflurane anaesthesia. If steady-state anaesthesia with isoflurane or enflurane has been established, the recommended initial Mivacron dose should be reduced by up to 25%. Halothane appears to have only a minimal potentiating effect on Mivacron and dose reduction of Mivacron is probably not necessary.
Once spontaneous recovery is underway it is complete in approximately 15 minutes and is independent of the dose of Mivacron administered.
The neuromuscular block produced by Mivacron can be reversed with standard doses of anticholinesterase agents. However, because spontaneous recovery after Mivacron is rapid, a reversal may not be routinely required as it shortens recovery time by only 5-6 minutes.
Use as an Infusion in Adults
Continuous infusion of Mivacron may be used to maintain neuromuscular block. Upon early evidence of spontaneous recovery from an initial Mivacron dose, an infusion rate of 8 to 10 micrograms/kg/min (0.5 to 0.6 mg/kg/hr) is recommended.
The initial infusion rate should be adjusted according to the patient’s response to peripheral nerve stimulation and clinical criteria. Adjustments of the infusion rate should be made and should be increments of approximately 1 microgram/kg/min (0.06 mg/kg/hr). In general, a given rate should be maintained for at least 3 minutes before a rate change is made. On average, an infusion rate of 6 to 7 micrograms/kg/minute will maintain neuromuscular block within the range of 89% to 99% for extended periods in adults receiving narcotic anaesthesia. During steady-state isoflurane or enflurane anaesthesia, reduction in the infusion rate by up to 40% should be considered. A study has shown that the mivacurium infusion rate requirement should be reduced by up to 50% with sevoflurane. With halothane, smaller reductions in infusion rate may be required.
Spontaneous recovery after Mivacron infusion is independent of the duration of infusion and comparable to recovery reported for single doses.
Continuous infusion of Mivacron has not been associated with the development of tachyphylaxis or cumulative neuromuscular blockade.
Mivacron (2 mg/ml) may be used undiluted for infusion.
Mivacron is compatible with the following infusion fluids.
Sodium chloride intravenous infusion (0.9% w/v)
Glucose intravenous infusion (5% w/v)
Sodium chloride (0.18% w/v) and glucose (4% w/v) intravenous infusion
Lactated Ringer’s Injection United States Pharmacopoeia (USP)
When diluted with the listed infusion solutions in the proportion of 1 plus 3 (i.e. to give 0.5 mg/ml) Mivacron injection has been shown to be chemically and physically stable for at least 48 hours at 30°C. However, since the product contains no antimicrobial preservative, dilution should be carried out immediately prior to use, administration should commence as soon as possible thereafter, and any remaining solution should be discarded.
Doses in Infants and Children Aged 2 Months – 12 Years
Mivacron has a faster onset, shorter clinically effective duration of action and more rapid spontaneous recovery profile in infants and children than in adults.
The ED95 in infants aged 2 to 6 months is approximately 0.07 mg/kg; and in infants and children aged 7 months to 12 years is approximately 0.1 mg/kg.
Pharmacodynamic data for recommended initial doses in infants and children are summarised in the following table:
| Age | Dose for Tracheal Intubation | Time to Maximum Neuromuscular Block (min) | Duration of Clinically Effective Block (min) |
|---|---|---|---|
| 2-6 monthsA | 0.15 mg/kg | 1.4 | 9 |
| 7 months-12 yearsB | 0.2 mg/kg | 1.7 | 9 |
A Data obtained during halothane anaesthesia.
B Data obtained during halothane or narcotic anaesthesia.
Since maximum block is usually achieved within 2 minutes following administration of these doses, tracheal intubation should be possible within this time.
Infants and children generally require more frequent maintenance doses and higher infusion rate than adults. Pharmacodynamic data for maintenance doses are summarised in the table below together with recommended infusion rates:
| Age | Maintenance Dose | Duration of Clinically Effective Block (min) | Average Infusion Rate Required to Maintain 89-99% Neuromuscular Block |
|---|---|---|---|
| 2 months-12 yearsA | 0.1 mg/kg | 6-9 | 11–14 mcg/kg/min (0.7-0.9 mg/kg/hr) |
A Data obtained during halothane or narcotic anaesthesia.
The neuromuscular blocking action of mivacurium is potentiated by inhalational agents. A study has shown that the mivacurium infusion rate requirement should be reduced by up to 70% with sevoflurane in children aged 2–12 years.
Once spontaneous recovery is underway, it is complete in approximately 10 minutes.
Dose in Neonates and Infants <2 Months of Age
The safety and efficacy of Mivacron in neonates and infants <2 months has not yet been established. Currently available data are described in section 5.1, but no recommendation on posology can be made.
Dose in the Elderly
In elderly patients receiving single bolus doses of Mivacron, the onset time, duration of action and recovery rate may be extended relative to younger patients by 20 to 30%. Elderly patients may also require decreased infusion rates or smaller or less frequent maintenance bolus doses.
Dose in Patients with Cardiovascular Disease
In patients with clinically significant cardiovascular disease, the initial dose of Mivacron should be administered over 60 seconds. Mivacron has been administered in this way with minimal haemodynamic effects to patients undergoing cardiac surgery.
Dose in Patients with Reduced Renal Function
In patients with end-stage renal failure the clinically effective duration of block produced by 0.15 mg/kg is approximately 1.5 times longer than in patients with normal renal function. Subsequently, dosage should be adjusted according to individual clinical response.
Prolonged and intensified neuromuscular blockade may also occur in patients with acute or chronic renal failure as a result of reduced levels of plasma cholinesterase (see section 4.4 Special Warnings and Precautions for Use).
Dose in Patients with Reduced Hepatic Function
In patients with end-stage liver failure the clinically effective duration of block produced by 0.15 mg/kg is approximately three times longer than in patients with normal hepatic function. This prolongation is related to the markedly reduced plasma cholinesterase activity seen in these patients. Subsequently, dosage should be adjusted according to individual clinical response.
Dose in Patients with Reduced Plasma Cholinesterase Activity
Mivacurium is metabolised by plasma cholinesterase. Plasma cholinesterase activity may be diminished in the presence of genetic abnormalities of plasma cholinesterase (e.g. patients heterozygous or homozygous for the atypical plasma cholinesterase gene), in various pathological conditions (see section 4.4 Special Warnings and Precautions for Use) and by the administration of certain drugs (see interactions with other medicaments). The possibility of prolonged neuromuscular block following administration of Mivacron must be considered in patients with reduced plasma cholinesterase activity. Mild reductions (i.e. within 20% of the lower limit of the normal range) are not associated with clinically significant effects on duration (See Contra-indications and Special Warnings and Precautions for information about use in patients who are homozygous or heterozygous for the plasma cholinesterase gene).
Dose in Obese Patients
In obese patients (those weighing 30% or more above their ideal bodyweight for height), the initial dose of Mivacron should be based upon ideal bodyweight and not actual bodyweight.
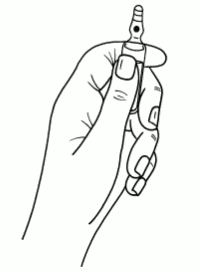
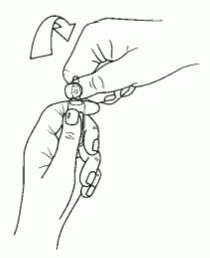
Instructions to open the ampoule
See Section 6.6 Special precautions for disposal and other handling for further information.
Monitoring
In common with all neuromuscular blocking agents, monitoring of neuromuscular function is recommended during the use of Mivacron in order to individualise dosage requirements.
Overdose
Prolonged muscle paralysis and its consequences are the main signs of overdosage with neuromuscular blocking agents. However, the risk of haemodynamic side-effects especially decreases in blood pressure may be increased.
It is essential to maintain a patent airway together with assisted positive pressure ventilation until spontaneous respiration is adequate. Full sedation will be required since consciousness is not impaired. Recovery may be hastened by the administration of anticholinesterase agents accompanied by atropine or glycopyrrolate, once evidence of spontaneous recovery is present. Cardiovascular support may be provided by proper positioning of the patient and administration of fluids or vasopressor agents as required.
Shelf life
18 months.
Special precautions for storage
Store below 25°C. Do not freeze. Protect from light.
Nature and contents of container
Neutral glass ampoules containing 5ml or 10ml of product.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Since no antimicrobial preservative is included, Mivacron must be used under full aseptic conditions and any dilution carried out immediately before use.
Any unused solution in open ampoules should be disposed of in accordance with local requirements.
Mivacron injection is acidic (approximately pH 4.5) and should not be mixed with highly alkaline solutions (e.g. barbiturates). Mivacron has been shown to be compatible with some commonly used peri-operative drugs supplied as acidic solutions. Where such agents are administered through the same indwelling needle or cannula as used for Mivacron injection, and compatibility has not been demonstrated, it is recommended that each drug is flushed through with physiological saline.
Instructions to open the ampoule
Ampoules are equipped with the OPC (One Point Cut) opening system and must be opened following the below instructions:
- hold with the hand the bottom part of the ampoule as indicated in picture 1
- put the other hand on the top of the ampoule positioning the thumb above the coloured point and press as indicated in picture 2
Picture 1:
Picture 2:
Administrative data.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.