SPIKEVAX Dispersion for injection Ref.[11078] Active ingredients: mRNA-1273
Source: European Medicines Agency (EU) Revision Year: 2021 Publisher: MODERNA BIOTECH SPAIN, S.L., Calle Monte Esquinza 30, 28010 Madrid, Spain
4.1. Therapeutic indications
Spikevax is indicated for active immunisation to prevent COVID-19 caused by SARS-CoV-2 in individuals 12 years of age and older.
The use of this vaccine should be in accordance with official recommendations.
4.2. Posology and method of administration
Posology
Primary series
Individuals 12 years of age and older
Spikevax is administered as a course of 2 (two) 100 microgram doses (0.5 mL each). It is recommended to administer the second dose 28 days after the first dose (see sections 4.4 and 5.1).
Booster dose
Individuals 18 years of age and older
A booster dose (0.25 mL, containing 50 micrograms mRNA, which is half of the primary dose) of Spikevax may be administered intramuscularly at least 6 months after the second dose in individuals 18 years of age and older. The decision when and for whom to implement a third dose of Spikevax should be made based on available vaccine effectiveness data, taking into account limited safety data (see sections 4.4 and 5.1).
The interchangeability of Spikevax with other COVID-19 vaccines to complete the primary vaccination course or the booster dose (0.25 mL, 50 micrograms) has not been established. Individuals who have received one dose of Spikevax (0.5 mL, 100 micrograms) should receive a second dose of Spikevax (0.5 mL, 100 micrograms) to complete the primary vaccination course.
Severely immunocompromised aged 12 years and older
A third dose (0.5 mL, 100 micrograms) may be given at least 28 days after the second dose to individuals who are severely immunocompromised (see section 4.4).
Paediatric population
The safety and efficacy of Spikevax in children and adolescents less than 12 years of age have not yet been established. No data are available.
Elderly population
No dosage adjustment is required in elderly individuals ≥65 years of age.
Method of administration
The vaccine should be administered intramuscularly. The preferred site is the deltoid muscle of the upper arm.
Do not administer this vaccine intravascularly, subcutaneously or intradermally.
The vaccine should not be mixed in the same syringe with any other vaccines or medicinal products.
For precautions to be taken before administering the vaccine, see section 4.4.
For instructions regarding thawing, handling and disposal of the vaccine, see section 6.6.
4.9. Overdose
No case of overdose has been reported.
In the event of overdose, monitoring of vital functions and possible symptomatic treatment is recommended.
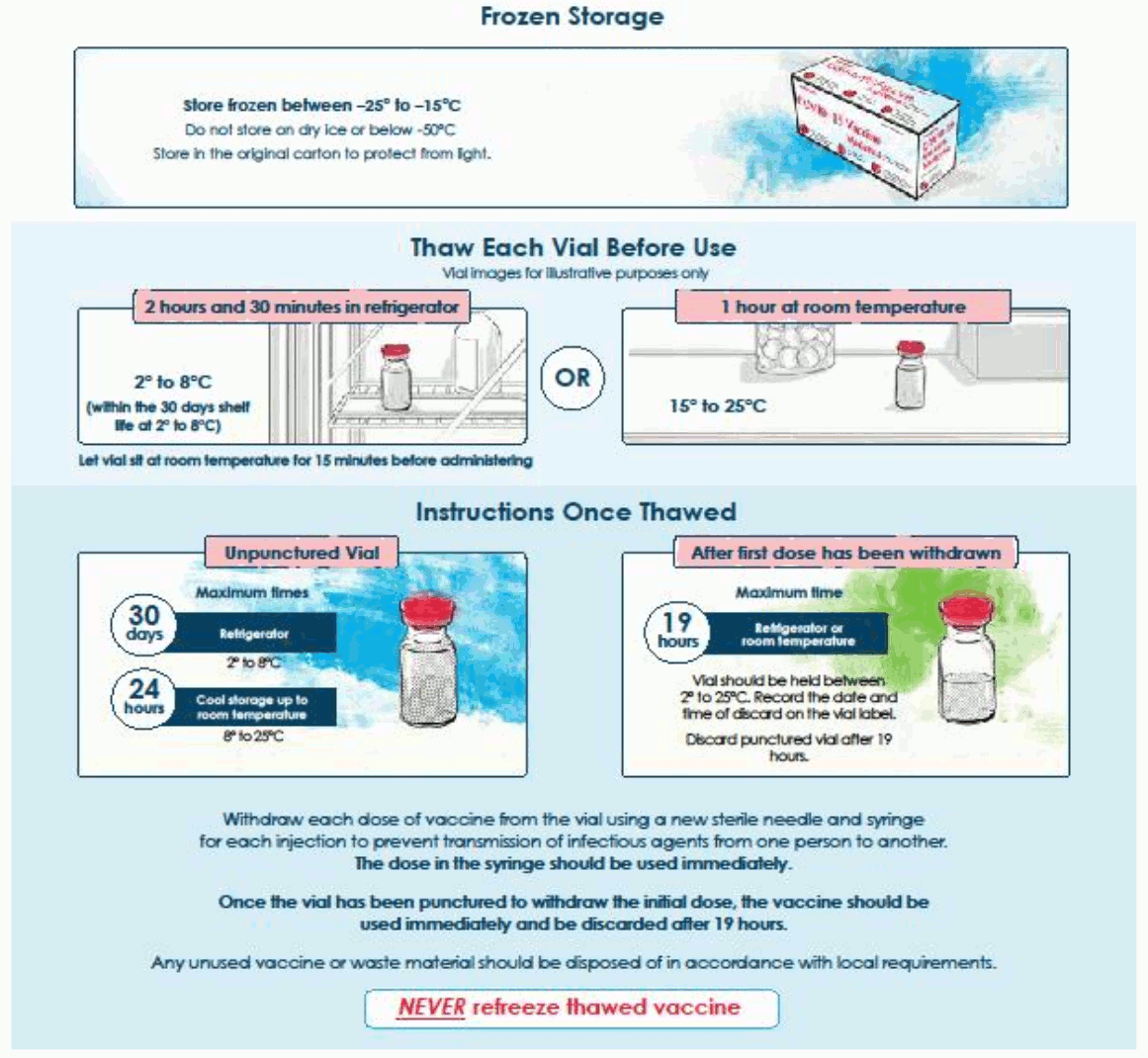
6.3. Shelf life
Unopened vial
7 months at -25ºC to -15ºC.
The unopened vaccine may be stored refrigerated at 2°C to 8°C, protected from light, for maximum 30 days. Within this period, up to 12 hours may be used for transportation.
Once thawed the vaccine should not be re-frozen.
The unopened vaccine may be stored at 8°C to 25°C up to 24 hours after removal from refrigerated conditions.
Punctured Vial
Chemical and physical in-use stability has been demonstrated for 19 hours at 2°C to 25ºC after initial puncture (within the allowed use period of 30 days at 2°C to 8ºC and 24 hours at 8°C to 25ºC). From a microbiological point of view, the product should be used immediately. If the vaccine is not used immediately, in-use storage times and conditions are the responsibility of the user.
6.4. Special precautions for storage
Store frozen between -25ºC to -15ºC.
Store in the original carton to protect from light.
Do not store on dry ice or below -50ºC.
For storage conditions after thawing and first opening see section 6.3.
Transportation of thawed vials in liquid state at 2°C to 8°C
If transport at -50°C to -15°C is not feasible, available data support transportation of one or more thawed vials in liquid state for up to 12 hours at 2°C to 8°C (within the 30 days shelf life at 2°C to 8°C). Once thawed and transported in liquid state at 2°C to 8°C, vials should not be refrozen and should be stored at 2°C to 8°C until use.
6.5. Nature and contents of container
5 mL dispersion in a vial (type 1 or type 1 equivalent glass) with a stopper (chlorobutyl rubber) and a flip-off plastic cap with seal (aluminium seal).
Each vial contains 5 mL.
Pack size: 10 multidose vials.
6.6. Special precautions for disposal and other handling
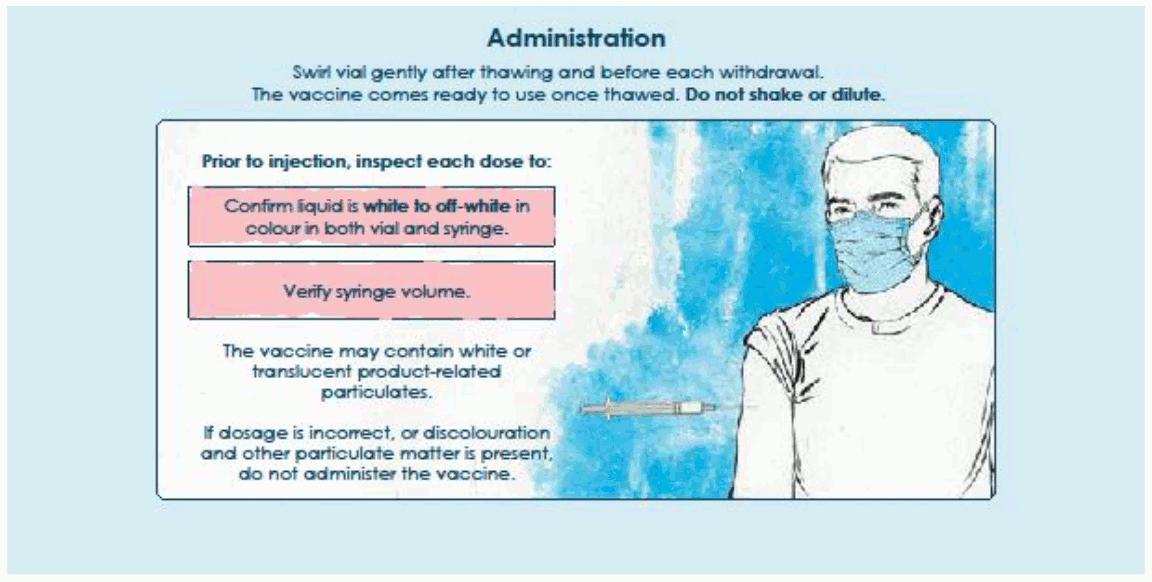
The vaccine should be prepared and administered by a trained healthcare professional using aseptic techniques to ensure sterility of the dispersion.
The vaccine comes ready to use once thawed.
Do not shake or dilute. Swirl the vial gently after thawing and before each withdrawal.
Spikevax vials are multidose.
Ten (10) doses (of 0.5 mL each) or a maximum of twenty (20) doses (of 0.25 mL each) can be withdrawn from each vial.
Pierce the stopper preferably at a different site each time. Do not puncture the vial more than 20 times.
An additional overfill is included in each vial to ensure that 10 doses of 0.5 mL or a maximum of 20 doses of 0.25 mL can be delivered.
Thawed vials and filled syringes can be handled in room light conditions.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.