MOXICAM Tablet Ref.[50024] Active ingredients: Meloxicam
Revision Year: 2022 Publisher: Alphapharm Pty Ltd trading as Viatris, Level 1, 30 The Bond, 30-34 Hickson Road, Millers Point NSW 2000 www.viatris.com.au Phone: 1800 274 276
Product name and form
MOXICAM.
| Pharmaceutical Form |
|---|
|
MOXICAM meloxicam 7.5 mg tablets: Each tablet contains 7.5 mg of meloxicam as the active ingredient, presented as a yellow coloured, circular 7 mm, flat bevelled uncoated tablet, with central break line on one side, plain on the other. MOXICAM meloxicam 15 mg tablets: Each tablet contains 15 mg of meloxicam as the active ingredient, presented as a pale yellow coloured, circular 10 mm, flat bevelled uncoated tablet, with central break line on one side, plain on the other. |
Qualitative and quantitative composition
Each MOXICAM tablet consists of 7.5 mg or 15 mg of the active ingredient meloxicam.
Excipient with known effect: lactose.
For the full list of excipients, see Section 6.1 LIST OF EXCIPIENTS.
Physicochemical properties
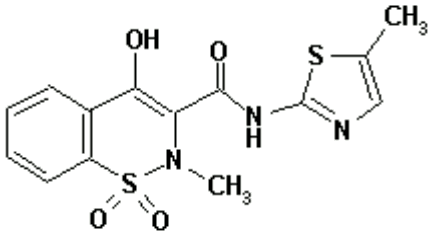
Chemical Structure
Chemical name: 4-hydroxy-2-methyl-N-(5-methyl-2-thiazolyl)-2H-1,2-benzothiazine-3-carboxamide-1,1-dioxide
Structural formula:
Molecular formula: C14H13N3O4S2
Molecular weight: 351.4
Meloxicam is a pastel yellow solid with pKa values of 1.09 and 4.18 and a melting point of about 256oC. The substance is practically insoluble in water, soluble in dimethylformamide, slightly soluble in chloroform and acetone, and very slightly soluble in methanol. There are no chiral centres and no polymorphs are formed under normal conditions.
CAS Number: 71125-38-7
| Active Ingredient |
|---|
|
Meloxicam is a non-steroidal anti-inflammatory drug (NSAID) of the oxicam family, with anti-inflammatory, analgesic and antipyretic properties. The anti-inflammatory activity of meloxicam has been proven in classical models of inflammation. As with other NSAID, its precise mechanism of action remains unknown. However, there is at least one common mode of action shared by all NSAID (including meloxicam): inhibition of the biosynthesis of prostaglandins, known inflammation mediators. |
| List of Excipients |
|---|
|
Pregelatinised maize starch, microcrystalline cellulose, lactose monohydrate, maize starch, sodium citrate dihydrate, colloidal anhydrous silica and magnesium stearate. |
Pack sizes and marketing
MOXICAM meloxicam 7.5 mg tablets: PVC/PVDC/Al blister packs of 10 and 30 tablets.
MOXICAM meloxicam 15 mg tablets: PVC/PVDC/Al blister packs of 10 and 30 tablets.
Some strengths, pack sizes and/or pack types may not be marketed.
Australian Register of Therapeutic Goods (ARTG):
AUST R 126214 – MOXICAM meloxicam 7.5 mg tablet blister pack.
AUST R 126215 – MOXICAM meloxicam 15 mg tablet blister pack.
Marketing authorization holder
Alphapharm Pty Ltd trading as Viatris, Level 1, 30 The Bond, 30-34 Hickson Road, Millers Point NSW 2000
www.viatris.com.au
Phone: 1800 274 276
Marketing authorization dates and numbers
Date of first approval: 11/05/2007
Drugs
| Drug | Countries | |
|---|---|---|
| MOXICAM | Australia, Hong Kong |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.