MVABEA Suspension for injection Ref.[50431] Active ingredients: Ebola vaccine
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Janssen-Cilag International NV, Turnhoutseweg 30, B-2340 Beerse, Belgium
4.1. Therapeutic indications
Mvabea, as part of the Zabdeno, Mvabea vaccine regimen, is indicated for active immunisation for prevention of disease caused by Ebola virus (Zaire ebolavirus species) in individuals ≥1 year of age (see sections 4.4 and 5.1).
The use of the vaccine regimen should be in accordance with official recommendations.
4.2. Posology and method of administration
Mvabea should be administered by a trained healthcare worker.
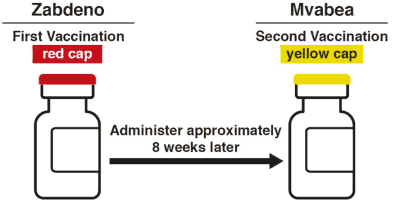
Mvabea is the second vaccination in the prophylactic 2-dose heterologous Ebola vaccine regimen which consists of vaccination with Zabdeno followed by a second vaccination with Mvabea given approximately 8 weeks later (see sections 4.4 and 5.1) (refer to the SmPC for Zabdeno).
Posology
Primary vaccination
A dose (0.5 mL) of Zabdeno (red cap vial) vaccine should be administered as the first vaccination (refer to the SmPC for Zabdeno).
A dose (0.5 mL) of Mvabea (yellow cap vial) vaccine should be administered as the second vaccination approximately 8 weeks after the first vaccination with Zabdeno.
Booster vaccination with Zabdeno (individuals who previously received the Zabdeno, Mvabea 2-dose primary vaccination regimen)
Individuals who have previously completed the 2-dose primary vaccination regimen can receive a booster dose of Zabdeno. As a precautionary measure, a Zabdeno booster vaccination is recommended in individuals who are at imminent risk of exposure to Ebola virus and have completed the 2-dose primary vaccination regimen more than 4 months ago (see sections 4.4 and 5.1).
Corrective measures in case of inadvertent administration
If Mvabea is inadvertently administered as the first vaccination, administration of Zabdeno is recommended as the second vaccination approximately 8 weeks later.
If Zabdeno is inadvertently administered as the first and the second vaccination, additional immunisation with Mvabea is recommended approximately 8 weeks after the second vaccination with Zabdeno.
If Mvabea is inadvertently administered as the first and the second vaccination, additional immunisation with Zabdeno is recommended approximately 8 weeks after the second vaccination with Mvabea.
If the second vaccination (Mvabea) of the regimen has been delayed beyond the recommended 8 weeks after the first vaccination (Zabdeno) of the regimen, the Mvabea vaccine should be administered regardless of the elapsed time from the first vaccination with Zabdeno (see section 5.1).
Paediatric population
The posology in children aged 1 to <18 years is the same as in adults. No data are available on the safety and efficacy of the 2-dose primary vaccination regimen and the booster vaccination in children aged <1 year.
Elderly population
No dosage adjustment is required in elderly individuals ≥65 years of age.
HIV-infected individuals
No dosage adjustment is required in HIV-infected individuals with infection controlled through antiretroviral therapy (see section 5.1).
Method of administration
Mvabea should be administered by the intramuscular (IM) route. The preferred site is the deltoid muscle of the upper arm. In younger children, either the deltoid region of the arm or anterolateral aspect of the thigh are acceptable sites for intramuscular injection.
Do not administer this vaccine intravenously or subcutaneously.
The vaccine should not be mixed in the same syringe with any other vaccines or medicinal products.
For precautions to be taken before administering the vaccine, see section 4.4.
For precautions regarding thawing, handling and disposal of the vaccine, see section 6.6.
4.9. Overdose
No case of overdose has been reported.
6.3. Shelf life
4 years at -85°C to -55°C
6.4. Special precautions for storage
Transport frozen at -25°C to -15°C. Upon receipt, the product can be stored as indicated below:
Store in a freezer at -85°C to -55°C at the distributor in case of stockpiling. The expiry date for storage at -85°C to -55°C is printed on the vial and outer carton after EXP.
The vaccine can also be stored by the distributor or end user in a freezer at -25°C to -15°C for a single period of up to 7 months. Upon removal from the -85°C to -55°C freezer, the new expiry date must be written by the distributor or end user on the outer carton and the vaccine should be used or discarded at the end of the 7 months. This new expiry date should not exceed the original expiry date (EXP). The original expiry date should be made unreadable.
The vaccine can also be stored by the distributor or end user in a refrigerator at 2°C to 8°C for a single period of up to 1 month. Upon moving the product to 2°C to 8°C storage, the discard date must be written by the distributor or end user on the outer carton and the vaccine should be used or discarded at the end of the 1 month period. This discard date should not exceed the original expiry date (EXP), or the new expiry date assigned for the -25°C to -15°C storage condition. The original expiry date and/or the new expiry date assigned for the -25°C to -15°C storage condition should be made unreadable.
Once thawed, the vaccine cannot be refrozen.
The vial must be kept in the original package in order to protect from light and to track the expiry or discard date for the different storage conditions.
6.5. Nature and contents of container
0.5 mL suspension in a single-dose Type I glass vial with a rubber stopper (chlorobutyl with fluoropolymer coated surface), aluminium crimp and yellow plastic cap.
Pack size of 20 single-dose vials.
6.6. Special precautions for disposal and other handling
Mvabea is a light yellow, clear to milky suspension. The vaccine should be inspected visually for particulate matter and discolouration prior to administration. The vial should be inspected visually for cracks or any abnormalities, such as evidence of tampering prior to administration. If any of these should exist, do not administer the vaccine.
Once the vaccine has been removed from the freezer and thawed, use immediately or store in a refrigerator at 2°C to 8°C (see section 6.4). Once removed from the refrigerator for administration, it should be used immediately.
Gently mix the contents of the vial by swirling for 10 seconds. Do not shake. Use a sterile needle and sterile syringe to extract the entire contents from the vial for administration.
Use a separate sterile needle and syringe for each individual. It is not necessary to change needles between drawing up the vaccine from a vial and injecting it into a recipient, unless the needle has been damaged or contaminated. Any remaining content in the vial should be discarded.
Any unused medicinal product or waste material should be disposed of in accordance to local requirements. Potential spills should be disinfected with agents with viricidal activity against vaccinia virus.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.