MYAMBUTOL Film-coated tablet Ref.[27454] Active ingredients: Ethambutol
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
MYAMBUTOL ethambutol hydrochloride is an oral chemotherapeutic agent which is specifically effective against actively growing microorganisms of the genus Mycobacterium, including M. tuberculosis.
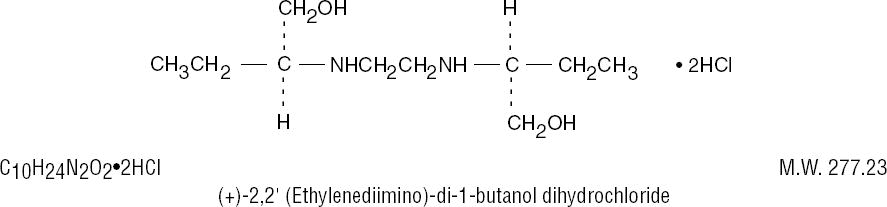
The structural formula is:
MYAMBUTOL 100 and 400 mg tablets contain the following inactive ingredients: Gelatin, Hydroxypropyl Methylcellulose, Magnesium Stearate, Sodium Lauryl Sulfate, Sorbitol, Stearic Acid, Sucrose, Titanium Dioxide and other ingredients.
| How Supplied |
|---|
|
MYAMBUTOL (Ethambutol Hydrochloride) Tablets USP 100 mg – round, convex, white, film coated tablets engraved with M6 on one side are supplied as follows: NDC 68850-010-01 – Bottle of 100 400 mg – round, convex, white, scored, film coated tablets engraved with M to the left and 7 to the right of the score on one side are NDC 68850-012-01 – Bottle of 100 Manufactured by Patheon Inc., Ontario, Canada. |
Drugs
| Drug | Countries | |
|---|---|---|
| MYAMBUTOL | Austria, Spain, France, Malta, Netherlands, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.