MYCOBUTIN Capsules Ref.[8365] Active ingredients: Rifabutin
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2015 Publisher: Pfizer Limited, Ramsgate Road, Sandwich, Kent, CT13 9NJ, United Kingdom
Contraindications
Hypersensitivity or history of hypersensitivity to the active substance, other rifamycins (e.g. rifampicin) or to any of the excipients listed in section 6.1.
Due to insufficient clinical experience in pregnant and breast-feeding women and in children, Mycobutin should not be used in these patients.
Special warnings and precautions for use
Before starting Mycobutin prophylaxis, patients should be assessed to ensure that they do not have active disease caused by pulmonary tuberculosis or other mycobacteria.
Prophylaxis against MAC infection may need to be continued throughout the patient's lifetime.
Mycobutin may impart a red-orange colour to the urine and possibly to skin and body secretions. Contact lenses, especially soft, may be permanently stained.
Mild hepatic impairment does not require a dose modification. Mycobutin should be used with caution in cases of severe liver insufficiency. Mild to moderate renal impairment does not require any dosage adjustment.
Severe renal impairment (creatinine clearance below 30 ml/min) requires a dosage reduction of 50%.
It is recommended that white blood cell and platelet counts and liver enzymes be monitored periodically during treatment.
Because of the possibility of occurrence of uveitis, patients should be carefully monitored when rifabutin is given in combination with clarithromycin (or other macrolides) and/or fluconazole (and related compounds). If such an event occurs, the patient should be referred to an ophthalmologist and, if considered necessary, Mycobutin treatment should be suspended.
Uveitis associated with Mycobutin must be distinguished from other ocular complications of HIV.
Clostridium difficile associated diarrhoea (CDAD) has been reported with use of nearly all antibacterial agents, including rifabutin, and may range in severity from mild diarrhoea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhoea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
Interaction with other medicinal products and other forms of interaction
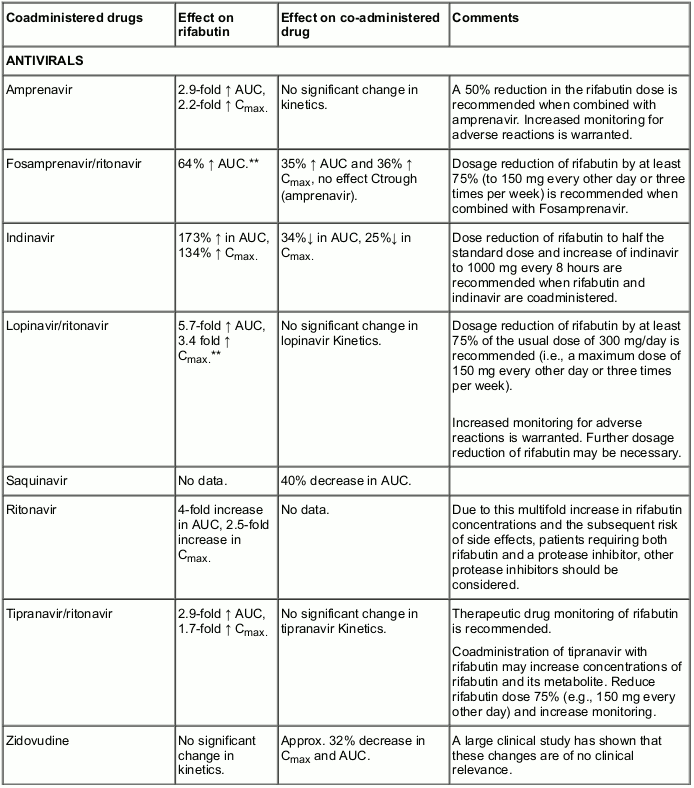
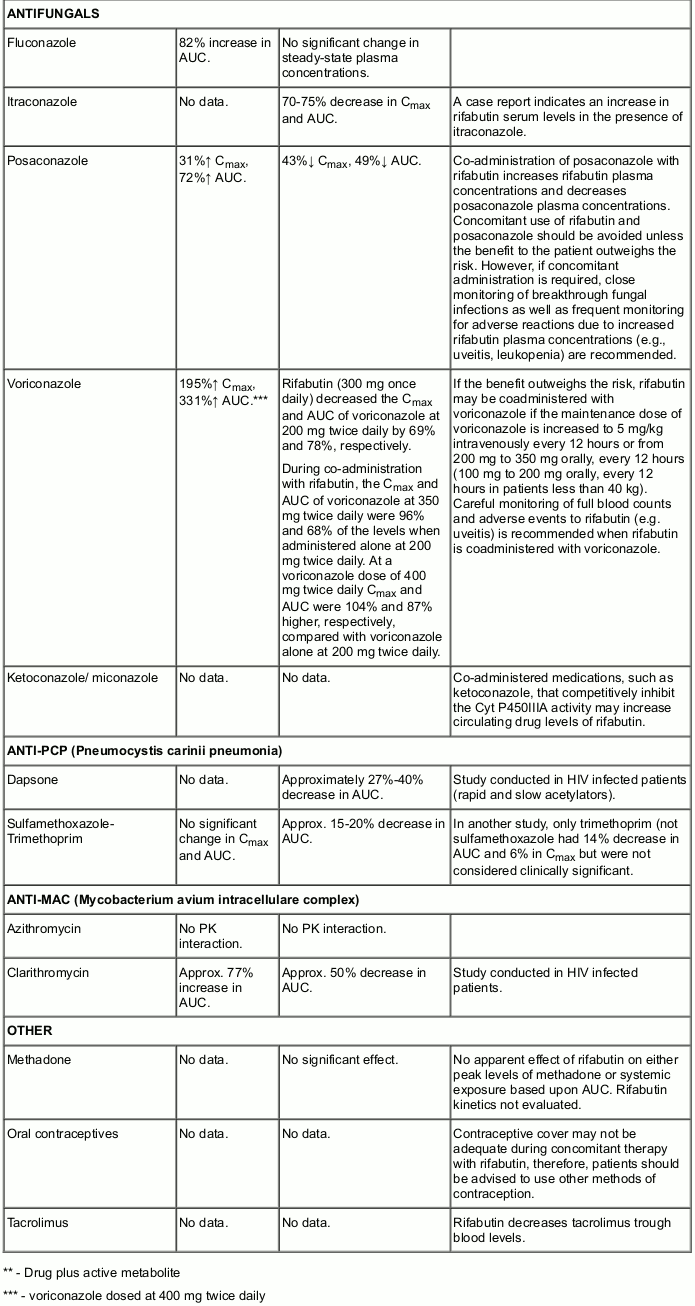
Rifabutin has been shown to induce the enzymes of the cytochrome P450 3A subfamily and therefore may affect the pharmacokinetic behaviour of drugs metabolised by the enzymes belonging to this subfamily. Upward adjustment of the dosage of such drugs may be required when administered with Mycobutin.
Similarly, Mycobutin might reduce the activity of analgesics, anticoagulants, corticosteroids, cyclosporin, digitalis (although not digoxin), oral hypoglycaemics, narcotics, phenytoin and quinidine.
Clinical studies have shown that Mycobutin does not affect the pharmacokinetics of didanosine (DDI), and isoniazid (however, for the latter refer also to undesirable effects). On the basis of the above metabolic considerations no significant interaction may be expected with ethambutol, theophylline, sulfonamides, pyrazinamide and zalcitabine (DDC).
As p-aminosalicylic acid has been shown to impede GI absorption of rifamycins it is recommended that when it and Mycobutin are both to be administered they be given with an interval of 8-12 hours.
The following table provides details of the possible effects of co-administration, on rifabutin and the co-administered drug, and risk-benefit statement.
Pregnancy and lactation
Due to lack of data in pregnant women, as a precautionary measure, Mycobutin should not be administered to pregnant women or those breast-feeding children even though in experimental animal studies the drug was not teratogenic.
Mycobutin may interact with oral contraceptives (see Section 4.5).
Effects on ability to drive and use machines
There have been no reports of adverse effects on ability to drive and use machines.
Undesirable effects
The tolerability of Mycobutin in multiple drug regimens, was assessed in both immunocompetent and immunocompromised patients, suffering from tuberculosis and non-tuberculous mycobacteriosis in long term studies with daily dosages up to 600 mg.
Bearing in mind that Mycobutin was often given in these studies as part of a multidrug regimen it is not always possible to define with certainty a drug-event relationship. Treatment discontinuation was necessary only in a very few cases. Adverse reactions identified through clinical trials or post-marketing surveillance by system organ class (SOC) are listed below in the following frequencies, very common ≥1/10; common ≥1/100 to <1/10; uncommon ≥1/1,000 to <1/100, rare ≥1/10,000 to <1/1,000, very rare <1/10,000 and 'not known'.
Blood and lymphatic system disorders
Very common: Leukopenia
Common: Anaemia
Uncommon: Pancytopenia, Agranulocytosis, Lymphopenia, Granulocytopenia, Neutropenia, White blood cell count decreased, Neutrophil count decreased, Thrombocytopenia, Platelet count decreased
Immune system disorders
Common: Rash
Uncommon: Hypersensitivity, Bronchospasm, Eosinophilia
Eye disorders
Uncommon: Uveitis, Corneal deposits
Gastrointestinal disorders
Common: Nausea
Uncommon: Vomiting
Hepatobiliary disorders
Uncommon: Jaundice, Hepatic enzyme increased
Skin and subcutaneous tissue disorders
Uncommon: Skin discolouration
Musculoskeletal and connective tissue disorders
Common: Myalgia
Uncommon: Arthralgia
General disorders and administration site conditions
Common: Pyrexia
Clostridium difficile colitis is a mandated adverse reaction for the pharmacological class; this event was neither observed in the clinical trials nor in the spontaneous reporting for rifabutin.
Anaphylactic shock has occurred with other antibiotics of the same class.
Mild to severe, reversible uveitis has been reported less frequently when Mycobutin is used at 300 mg as monotherapy in MAC prophylaxis, versus Mycobutin in combination with clarithromycin (or other macrolides) for MAC treatment (see Section 4.4).
Flu-like syndrome, chest pressure or pain with dyspnoea and rarely hepatitis and haemolysis has been reported.
Reporting of suspected adverse reactions
Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the Yellow Card Scheme at: www.mhra.gov.uk/yellowcard.
Incompatibilities
Not applicable.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.