MYONAL Tablets / Granules Ref.[11086] Active ingredients: Eperisone
Revision Year: 2009
Product description
Tablets 50 mg
Each white, sugar-coated tablet contains 50 mg of eperisone hydrochloride.
It contains carnauba wax, carmellose, hydrated silicon dioxide, microcrystalline cellulose, titanium oxide, stearic acid, calcium stearate, sucrose, talc, precipitated calcium carbonate, corn starch, white shellac, hydroxypropylcellulose, pullulan, povidone and macrogol 6000 as inactive ingredients.
Granules 10%
Each gram of white to yellowish white granules contains 100 mg of eperisone hydrochloride.
It contains carmellose, light anhydrous silicic acid, talc, corn starch, lactose hydrate, povidone, polyvinylacetal diethylaminoacetate and macrogol 6000 as inactive ingredients.
Physicochemistry
Nonproprietary name: Eperisone Hydrochloride (JAN), Eperisone (INN)
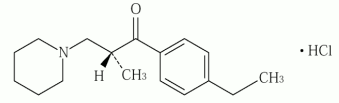
Chemical name: (2RS)-1-(4-Ethylphenyl)-2-methyl-3-piperidin-1-ylpropan-1-one monohydrochloride
Molecular formula: C17H25NO ⋅ HCl
Molecular weight: 295.85
Structural formula:
Description: Eperisone hydrochloride occurs as a white, crystalline powder. It is freely soluble in water, in methanol and in acetic acid (100), soluble in ethanol (99.5). A solution of eperisone hydrochloride in methanol (1 in 100) shows no optical rotation.
Melting point: About 167°C (decomposition)
| How Supplied |
|---|
|
MYONAL Tablets 50 mg: Boxes of 100, 210 (21 Tabs. × 10), 1,000, 1,050 (21 Tabs. × 50), 3,000 and 3,150 (21 Tabs. × 150) in press-through packages, and bottles of 500. MYONAL Granules 10%: Cans of 100 g. Manufactured and marketed by: Eisai Co., Ltd. 6-10, Koishikawa 4-chome, Bunkyo-ku, Tokyo, 112-8088 |
Drugs
| Drug | Countries | |
|---|---|---|
| MYONAL | Japan, Singapore |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.