MYSOLINE Tablet Ref.[50489] Active ingredients: Primidone
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
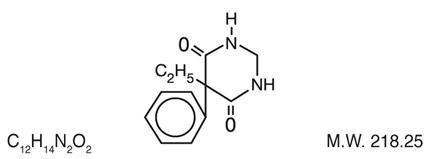
Chemical name: 5-ethyldihydro-5-phenyl-4,6 (1H, 5H)-pyrimidinedione. Structural formula:
MYSOLINE (primidone) is a white, crystalline, highly stable substance, M.P. 279-284°C. It is poorly soluble in water (60 mg per 100 mL at 37°C) and in most organic solvents. It possesses no acidic properties, in contrast to its barbiturate analog.
MYSOLINE 50 mg and 250 mg tablets contain the following inactive ingredients: lactose monohydrate, NF; magnesium stearate, NF; methylcellulose, USP; microcrystalline cellulose, NF; purified water, USP; sodium lauryl sulfate, NF; sodium starch glycolate, NF; and talc, USP.
MYSOLINE 250 mg tablets also contain ferric oxide yellow, NF.
| How Supplied |
|---|
|
MYSOLINE Tablets: Modified square, flat faced, beveled edge, compressed light yellow color tablet. One face is debossed (impressed) with "MYSOLINE" and "250" that are divided by a debossed bisect line. The opposite side is embossed with the letter "M", in bottles of 100 (NDC 66490-691-10). Modified square, flat faced, beveled edge, compressed white color tablet. One face is debossed (impressed) with "MYSOLINE" and "50" that are divided by a debossed bisect line. The opposite side is embossed with the letter "M", in bottles of 100 (NDC 66490-690-10). Distributed by: Bausch Health US, LLC, Bridgewater, NJ 08807 USA Manufactured by: Bausch Health Companies Inc., Steinbach, MB R5G 1Z7, Canada |
Drugs
| Drug | Countries | |
|---|---|---|
| MYSOLINE | Austria, Australia, Spain, France, Ireland, Malta, Mexico, Netherlands, Turkey, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.