NADROPARIN SODIUM Solution for injection Ref.[9376] Active ingredients: Nadroparin
Source: European Medicines Agency (EU) Publisher: Aspen Pharma Trading Limited, 3016 Lake Drive, Citywest Business Campus, Dublin 24, Ireland
Therapeutic indications
Treatment of deep venous thrombosis.
Posology and method of administration
Posology
Treatment of deep venous thrombosis
Nadroparin 19,000 I.U. should be injected subcutaneously once daily for the duration of 10 days in a dosage appropriately adjusted for the patient's body weight (see Table below).
The administration of oral anticoagulants should be started on the first day. The duration of treatment with Nadroparin 19,000 I.U. is at least 5 days and should be continued until sufficient oral anticoagulation has been achieved.
In patients with thrombophilia or complicated deep venous thrombosis, or in patients with increased risk of bleeding complications, a twice-daily administration of nadroparin calcium should be considered (see Fraxiparine).
The prefilled syringes are graduated in 0.1 ml increments. For patients needing dosages of 0.4 ml, 0.5 ml, 0.7 ml or 0.9 ml, in accordance with their individual body weight, the correct dosage can be obtained by using the respective higher-dose prefilled syringe and discarding the excess amount of 0.1 or 0.2 ml before use.
Treatment of deep venous thrombosis
| Weight in kg | Treatment of deep venous thrombosis ml subcutaneous injection once daily | ||
|---|---|---|---|
| <50 | 0.4 ml | ||
| 50 to 59 | 0.5 ml | 60 to 69 | 0.6 ml |
| 70 to 79 | 0.7 ml | ||
| 80 to 89 | 0.8 ml | ||
| ≥90 | 0.9 ml |
Monitoring during treatment
Because of the risk of heparin-induced thrombocytopenia, the platelet count must be monitored regularly during treatment with Nadroparin 19,000 I.U.
Checking the platelet count is recommended prior to initiating treatment, during the first day of treatment and subsequently every 3 to 4 days, as well as at the end of treatment.
Occasionally, a mild transient thrombocytopenia (Type I) with platelet counts between 100,000/microliter and 150,000/microliter (caused by transient platelet activation) occurs at the beginning of treatment. Complications generally do not occur in these cases. Treatment may, therefore, be continued.
Antibody-mediated severe thrombocytopenia (Type II) with platelet counts clearly below 100,000/microliter or a rapid drop to less than 50% of the initial value is rarely observed. In non-sensitized patients, platelet count decrease primarily starts 6 to 21 days after the onset of treatment; in sensitized patients, platelet count decrease may start within hours. The severe form of thrombocytopenia may be associated with arterial and venous thrombosis/thromboembolism, disseminated intravascular coagulation, and possible skin necrosis at the injection site, petechiae, purpura and melena. In such cases, Nadroparin 19,000 I.U. must be discontinued immediately and a different antithrombotic treatment must be considered. The patient must be informed that he or she may no longer use heparin-containing medications in the future.
Paediatric population
Nadroparin is not recommended in children and adolescents, as there are insufficient data on safety and efficacy to determine dosages for patients younger than 18 years of age.
Elderly patients
Dosage adjustment in elderly patients is not necessary, except in case of kidney disorders. It is recommended to monitor the kidney function in elderly patients prior to starting treatment (see Impaired renal function below and under section 5.2).
Patients with impaired hepatic function
There have been no studies conducted in patients with hepatic impairment.
Impaired renal function
Moderate and severe renal impairment is associated with increasing exposure to nadroparin. These patients are at an increased risk of thromboembolism and haemorrhage.
If patients with renal impairment (see section 4.3) are treated for deep venous thrombosis, the laboratory test results should be monitored, preferably via anti-Xa level determination (amidolytic method with chromogenic substrate). The anti-Xa activity can be checked during the 2nd and the 4th day, 4 to 6 hours following subcutaneous application. Anti-Xa values above 1.8 I.U./ml may be an indication of an overdose and should lead to a dose reduction.
Nadroparin is contraindicated in patients with severe renal impairment (creatinine clearance below 30 ml/min) (see section 4.3, Contraindications).
Method of administration
For subcutaneous administration of Nadroparin 19,000 I.U., the lateral abdominal wall is the usual site of injection. As an alternative, Nadroparin 19,000 I.U. can be injected into the thigh.
The needle is inserted perpendicularly into a fold of skin formed between the thumb and index finger that should be gently but firmly held until injection has been completed. The injection site should not be rubbed.
Overdose
Symptoms and Signs
In the treatment of deep venous thrombosis, prolongation of the activated Partial Thromboplastin Time (aPTT) value should only be considered as an indication of overdose. Dose-escalations aiming at aPTT prolongation bear the risk of an overdose or bleeding. Haemorrhage is the major sign of overdose. Monitoring of platelet count and other coagulation parameters is advised.
Treatment
Minor bleeding rarely requires specific treatment. It often suffices to reduce or delay the next nadroparin dose. The administration of protamine sulphate should only be considered if the condition of the patient is serious.
It largely neutralises the anticoagulant effect of nadroparin but some anti-Xa activity will remain. 0.6 ml of protamine sulphate neutralises about 950 I.U. anti-Xa nadroparin. The amount of protamine to be injected, should take into account time elapsed from the injection of heparin, and a dose reduction of protamine may be appropriate.
Shelf life
3 years.
Special precautions for storage
Store below 25°C.
Nature and contents of container
The prefilled syringe consists of a Type-I glass cylinder with stainless steel needle and needle guard made of natural and/or styrene-butadiene-rubber, a safety cylinder made of polypropylene and a piston with sealing lip made of butyl elastomer.
Packs with 2, 6, 10, 20, 30 and 50 graduated prefilled syringes, each containing 0.8 ml injection solution (0.8 ml injection solution contains 15,200 I.U. anti-Xa nadroparin calcium).
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
Prior to administration, visually inspect for particulate matter and discoloration. Discard the injection
solution if any visual change has occurred. For single use only. Discard any unused solution.
Any unused medicinal product or waste material should be disposed of in accordance with local
requirements.
Step by step guide:
Parts of the NADROPARINE CALCIUM prefilled syringe:
- Needle guard
- Piston
- Syringe handle
- Safety sleeve
Instructions for use
1. Wash your hands thoroughly with soap and water and then dry them with a towel.
2. Take the syringe out of the carton and check:
- the expiration date located on the outer carton and on the pre-filled syringe
- if the syringe is opened or damaged
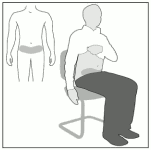
3. Sit or lie down comfortably
Select an area of skin in the lower abdominal region, at least 5 cm below the navel (Figure A).
Alternate the left and right injection site in the lower abdominal region at each injection. This helps to reduce possible discomfort at the injection site. If it is not possible to inject into the lower abdominal region, ask your doctor for advice.
Figure A:
4. Clean the injection area with an alcohol swab
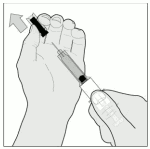
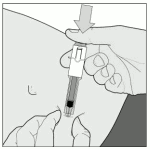
5. Remove the needle guard, by turning it and then pulling it in a straight line from the syringe body (Figure B).
Discard the needle guard.
If the volume of the syringe is greater than you need, you must remove the excess before you inject.
- Hold the syringe vertical so that the needle is pointing downwards.
- Push the piston gently downward until the bottom of the trapped air bubble sits at the mark with the volume that your doctor has prescribed for you.
- Allow the liquid that comes out of the needle to drop onto a tissue and discard it.
- The syringe is now ready for use.
Figure B:
Important note:
- Do not touch the needle or allow it to come into contact with anything before the injection.
- The presence of an air bubble in the prefilled syringe is normal. Do not try to remove this air bubble before performing the injection – you could otherwise lose some of the medicine.
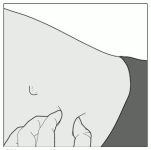
6. Gently pinch the skin that has been cleaned to make a fold. Hold this fold between thumb and index finger during the entire injection (Figure C.)
Figure C:
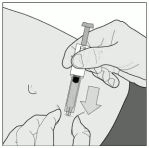
7. Hold the syringe firmly by the syringe handle. Insert the full length of the needle at a right angle into the skin fold (Figure D).
Figure D:
8. Inject ALL of the content in the prefilled syringe under the skin by pushing the plunger down as far as possible (Figure E). Remove the syringe gently from the skin.
Figure E:
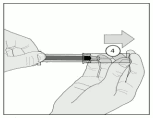
9. After injection, hold the prefilled syringe by the safety sleeve with one hand. Use the other hand to firmly pull the syringe back. This unlocks the cylinder. Slide the cylinder over the syringe until it locks into place over the needle (Figure F).
Figure F:
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.