NEBIDO Solution for injection Ref.[7121] Active ingredients: Testosterone
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2018 Publisher: Bayer plc, 400 South Oak Way, Reading, RG2 6AD
Therapeutic indications
Testosterone replacement therapy for male hypogonadism when testosterone deficiency has been confirmed by clinical features and biochemical tests (see section 4.4).
Posology and method of administration
Posology
One ampoule/vial of Nebido (corresponding to 1000 mg testosterone undecanoate) is injected every 10 to 14 weeks. Injections with this frequency are capable of maintaining sufficient testosterone levels and do not lead to accumulation.
Start of treatment
Serum testosterone levels should be measured before start and during initiation of treatment. Depending on serum testosterone levels and clinical symptoms, the first injection interval may be reduced to a minimum of 6 weeks as compared to the recommended range of 10 to 14 weeks for maintenance. With this loading dose, sufficient steady state testosterone levels may be achieved more rapidly.
Maintenance and individualisation of treatment
The injection interval should be within the recommended range of 10 to 14 weeks. Careful monitoring of serum testosterone levels is required during maintenance of treatment. It is advisable to measure testosterone serum levels regularly. Measurements should be performed at the end of an injection interval and clinical symptoms considered. These serum levels should be within the lower third of the normal range. Serum levels below normal range would indicate the need for a shorter injection interval. In case of high serum levels an extension of the injection interval may be considered.
Special populations
Paediatric population
Nebido is not indicated for use in children and adolescents and it has not been clinically evaluated in males under 18 years of age (see section 4.4).
Geriatric patients
Limited data do not suggest the need for a dosage adjustment in elderly patients (see section 4.4).
Patients with hepatic impairment
No formal studies have been performed in patients with hepatic impairment. The use of Nebido is contraindicated in men with past or present liver tumours (see section 4.3).
Patients with renal impairment
No formal studies have been performed in patients with renal impairment.
Method of administration
For intramuscular use.
The injections must be administered very slowly (over two minutes). Nebido is strictly for intramuscular injection. Care should be taken to inject Nebido deeply into the gluteal muscle following the usual precautions for intramuscular administration. Special care must be taken to avoid intravasal injection (see section 4.4 under “Application”). The contents of an ampoule/vial are to be injected intramuscularly immediately after opening. (For the ampoule see section 6.6 for instructions on opening the ampoule safely).
Overdose
No special therapeutic measure apart from termination of therapy with the medicinal product or dose reduction is necessary after overdose.
Shelf life
Shelf life: 5 years.
The medicinal product must be used immediately after first opening.
Special precautions for storage
This medicinal product does not require any special storage conditions.
Nature and contents of container
Ampoule: 5-ml brown glass (type I) ampoules, containing a fill volume of 4 ml.
Pack size: 1 × 4 ml.
Vial: 6ml brown glass (type I) vial with grey bromobutyl (foil-clad ETFE) injection stopper and bordered cap, containing a fill volume of 4 ml.
Pack size: 1 × 4 ml.
Special precautions for disposal and other handling
At cold storage temperatures the properties of this oil-based solution might temporarily change (e.g. higher viscosity, cloudiness). If stored at cold temperature, the product should be brought to room or body temperature before use.
The solution for intramuscular injection is to be visually inspected prior to use and only clear solutions free from particles should be used.
The medicinal product is for single use only and any unused solution should be discarded in accordance with local requirements.
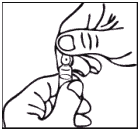
Ampoule:
Notes on handling the OPC (One-Point-Cut) ampoule: There is a pre-scored mark beneath the coloured point on the ampoule eliminating the need to file the neck. Prior to opening, ensure that any solution in the upper part of the ampoule flows down to the lower part. Use both hands to open; while holding the lower part of the ampoule in one hand, use the other hand to break off the upper part of the ampoule in the direction away from the coloured point.
Vial:
The vial is for single use only. The content of a vial is to be injected intramuscularly immediately after drawing up into the syringe. After removal of the plastic cap (A) do not remove the metal ring (B) or the crimp cap (C).
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.