NEULASTIM Solution for injection Ref.[50344] Active ingredients: Pegfilgrastim
Source: Health Products Regulatory Authority (ZA) Revision Year: 2015 Publisher: Amgen South Africa (Pty) Ltd. Building D, Ballyoaks Office Park, 35 Ballyclare Drive, Bryanston Ext. 7, South Africa
5.1. Pharmacodynamic properties
A 8.5 Medicines acting on blood and haemopoietic system – others.
Pharmacodynamic properties
Human granulocyte colony stimulating factor (G-CSF) is a glycoprotein, which regulates the production and release of neutrophils from the bone marrow. Pegfilgrastim is a covalent conjugate of recombinant human G-CSF (r-metHuG-CSF) with a single 20 kDa polyethylene glycol (PEG) molecule. Pegfilgrastim is a sustained duration form of filgrastim due to decreased renal clearance. A transient increase in the white cell count is the expected consequence of pegfilgrastim administration. Pegfilgrastim and filgrastim have been shown to have identical modes of action, causing a marked increase in peripheral blood neutrophil counts within 24 hours, with minor increases in monocytes and/or lymphocytes. Neutrophils produced in response to pegfilgrastim show normal or enhanced function as demonstrated by tests of chemotactic and phagocytic function. G-CSF has shown in vitro stimulating properties on human endothelial cells. G-CSF can promote growth of myeloid cells, including malignant cells in vitro, and similar effects may be seen on some non-myeloid cells in vitro.
5.2. Pharmacokinetic properties
Absorption
After a single subcutaneous dose of pegfilgrastim, the peak serum concentration of pegfilgrastim occurs at 16 to 120 hours after dosing.
Distribution
Serum concentrations of pegfilgrastim are maintained during the period of neutropenia after myelosuppressive chemotherapy. The distribution of pegfilgrastim was limited to the plasma compartment.
Elimination
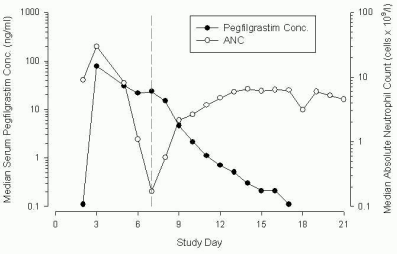
The elimination of pegfilgrastim is non-linear with respect to dose; serum clearance of pegfilgrastim decreases with increasing dose. Pegfilgrastim appears to be mainly eliminated by neutrophil mediated clearance (>99%), which becomes saturated at higher doses. Consistent with a self-regulating clearance mechanism, the serum concentration of pegfilgrastim declines rapidly at the onset of neutrophil recovery (see Figure 1). Figure 1: Profile of median pegfilgrastim serum concentration and Absolute Neutrophil Count (ANC) in chemotherapy-treated patients after a single 6 mg injection.
Figure 1: Profile of median pegfilgrastim serum concentration and Absolute Neutrophil Count (ANC) in chemotherapy-treated patients after a single 6 mg injection:
Pharmacokinetics in Special Populations
Due to the neutrophil-mediated clearance mechanism, the pharmacokinetics of pegfilgrastim is not expected to be affected by renal or hepatic impairment. Limited data indicate that the pharmacokinetics of pegfilgrastim in elderly subjects (> 65 years) is similar to that in adults.
Paediatrics
The pharmacokinetics of NEULASTIM were studied in 37 paediatric patients with sarcoma. The systemic exposure (AUC0-inf, mean ± Standard Deviation) of NEULASTIM after subcutaneous administration at 100 µg/kg was 22,0 (± 13,1) µg·hr/ml in the 6-11 years age group (n = 10), 29,3 (± 23,2) µg·hr/ml in the 12-21 years age group (n = 13) and 47,9 (± 22,5) µg·hr/ml in the youngest age group (0-5 years, n = 11 ). The terminal elimination half-lives of the corresponding age groups were 20,2 (± 11,3) hours, 21,2 (± 16,0) hours and 30,1 (± 38,2) hours, respectively. Note that the systemic exposure is much higher and the T1/2, longer in children aged 0-6 years. See DOSAGE and DIRECTIONS FOR USE, SIDE-EFFECTS AND SPECIAL PRECAUTIONS.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.