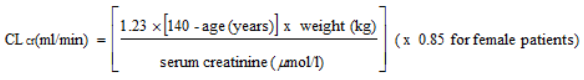NEURALYN Capsule, hard Ref.[115175] Active ingredients: Pregabalin
Source: Health Products Regulatory Authority (ZA) Revision Year: 2024 Publisher: Unicorn Pharmaceuticals (Pty) Ltd, Cnr. Searle & Pontac Streets, Cape Town, South Africa, 8001
Therapeutic indications
NEURALYN capsules are indicated for the treatment of adult patients with neuropathic pain due to Herpes zoster infections and diabetes.
Posology and method of administration
Posology
The recommended starting dose for NEURALYN is 75 mg twice daily (150 mg/day), with or without food. Based on individual patient response and tolerability, the dose may be increased to 150 mg twice daily after an interval of 3 to 7 days. In accordance with current clinical practice, if NEURALYN has to be discontinued, it is recommended this should be done gradually over a minimum of 1 week.
Special Populations
Patients with renal impairment
NEURALYN is eliminated from the systemic circulation primarily by renal excretion as unchanged pregabalin. As NEURALYN clearance is directly proportional to creatinine clearance (see section 5.2), dosage reduction in patients with compromised renal function must be individualised according to creatinine clearance (CLcr), as indicated in Table 1 determined using the following formula:
Table 1. NEURALYN dosage adjustment based on renal function1:
| Creatinine clearance (CLCR) (ml/min) | Total NEURALYN daily dose* | Dose regimen | |
|---|---|---|---|
| Starting dose (mg/day) | Maximum dose (mg/day) | ||
| ≥60 | 150 | 300 | BD |
| 30-60 | 75 | 150 | OD or BD |
| 15-30 | 25-50 | 75 | OD or BD |
| <15 | 25 | 25-50 | OD |
| Supplementary dosage following haemodialysis (mg) | |||
| 25 | 50 | Single dose' | |
BD = Two divided doses
OD = Once daily
* Total daily dose (mg/day) should be divided as indicated by dose regimen to
provide mg/dose
' Supplementary dose is a single additional dose
NEURALYN is removed effectively from plasma by haemodialysis (50 % of medicine in 4 hours). For patients receiving haemodialysis, the NEURALYN daily dose should be adjusted based on renal function. In addition to the daily dose, a supplementary dose should be given immediately following every 4-hour haemodialysis treatment (see Table 1).
Use in patients with hepatic impairment
No dosage adjustment is required for patients with hepatic impairment (see section 5.2).
Paediatric patients
The safety and effectiveness of NEURALYN in patients below the age of 18 years with neuropathic pain has not been established.
Use in the elderly (over 65 years of age)
No dosage adjustment is necessary for elderly patients unless their renal function is compromised, see Table 1.
Method of administration
NEURALYN is given orally with or without food.
Overdose
In the post marketing experience, the most commonly reported adverse reactions observed when pregabalin was taken in overdose included affective disorder, somnolence, confusional state, agitation, depression and restlessness. Seizures were also reported. In rare occasions, cases of coma have been reported.
Treatment of pregabalin overdose should include general supportive measures and may include haemodialysis if necessary (see section 4.2 Table 1).
Shelf life
36 months.
Special precautions for storage
No special temperature storage conditions are required.
Store in the original packaging.
Keep the container well closed.
Nature and contents of container
This medicine is packaged in a cardboard box containing PVC-Aluminum foil blisters or in white opaque HDPE bottles with child resistant screw caps.
Blister pack sizes of: 14, 56, 60 or 100 (blisters of 7 or 10 capsules), and
HDPE bottles containing 60 hard capsules.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
No special requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.