NEXPLANON Implant Ref.[8436] Active ingredients: Etonogestrel
Source: Medicines & Healthcare Products Regulatory Agency (GB) Revision Year: 2020 Publisher: Merck Sharp & Dohme Limited, Hertford Road, Hoddesdon, Hertfordshire, EN11 9BU
Therapeutic indications
Contraception.
Safety and efficacy have been established in women between 18 and 40 years of age.
Posology and method of administration
Posology
1 implant, which can be left in place for three years.
Paediatric population
The safety and efficacy of Nexplanon in adolescents under the age of 18 have not been established.
Method of administration
Pregnancy should be excluded before insertion of Nexplanon.
It is strongly recommended that Nexplanon be inserted and removed only by healthcare professionals (HCPs) who have completed training for the use of the Nexplanon applicator and the techniques for insertion and removal of the Nexplanon implant, and, where appropriate, that supervision be requested prior to inserting or removing the implant.
Before to inserting the implant, carefully read and follow the instructions for insertion and removal of the implant in section 4.2 How to insert Nexplanon and How to remove Nexplanon.
Videos demonstrating insertion and removal of the implant are available online for training purposes at www.nexplanonvideos.eu
Please contact your local representative of the Marketing Authorisation Holder if you have any questions or require further re-training.
Merck Sharp & Dohme Limited, Hertford Road, Hoddesdon, Hertfordshire, EN11 9BU, Telephone: 01992 467272
If you are unsure of the necessary steps to safely insert and/or remove Nexplanon, do not attempt the procedure.
How to use Nexplanon
Nexplanon is a long-acting hormonal contraceptive. A single implant is inserted subdermally and can be left in place for three years. Remove the implant no later than three years after the date of insertion. The user should be informed that she can request the removal of the implant at any time. HCPs may consider earlier replacement of the implant in heavier women (see section 4.4). After the removal of the implant, immediate insertion of another implant will result in continued contraceptive protection. If the woman does not wish to continue using Nexplanon, but wants to continue preventing pregnancy, another contraceptive method should be recommended.
The Nexplanon package contains a Patient Alert Card intended for the woman which records the batch number of the implant. HCPs are requested to record the date of insertion, the arm of insertion and the intended date of removal on the Patient Alert Card. Patients should be instructed to keep the Patient Alert Card in a safe place and show the Card at any visits related to the use of her implant. The Patient Alert Card also contains instructions for the patient to occasionally gently palpate the implant to be sure that she knows its location. Patients should be instructed to contact their doctor as soon as possible if at any time they cannot feel the implant. The package also includes adhesive labels intended for HCP records showing the batch number. This information should be included in the electronic medical records of the patient if such are used.
The basis for successful use and subsequent removal of the Nexplanon implant is a correct and carefully performed subdermal insertion of the implant in accordance with the instructions.
- If the implant is not inserted in accordance with the instructions and not on the correct day, this may result in an unintended pregnancy (see section 4.2 How to insert Nexplanon and When to insert Nexplanon).
- An implant inserted more deeply than subdermally (deep insertion) may not be palpable and the localisation and/or removal can be difficult (see section 4.2 How to remove Nexplanon and section 4.4).
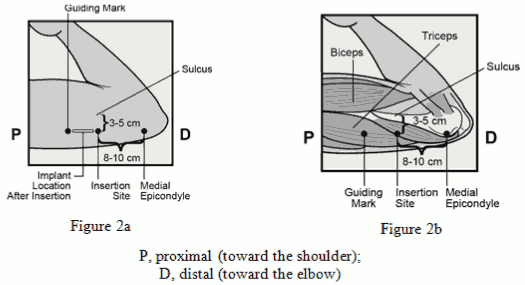
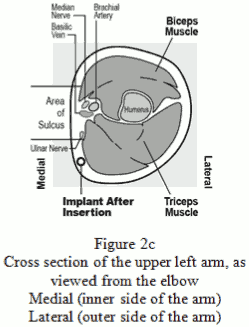
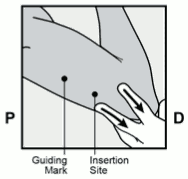
The Nexplanon implant should be inserted subdermally JUST UNDER THE SKIN at the inner side of the non-dominant upper arm. The insertion site is overlying the triceps muscle about 8-10 cm (3-4 inches) from the medial epicondyle of the humerus and 3-5 cm (1.25-2 inches) posterior to (below) the sulcus (groove) between the biceps and triceps muscles. This location is intended to avoid the large blood vessels and nerves lying within and surrounding the sulcus (see Figures 2a, 2b and 2c).
Immediately after insertion, the presence of the implant should be verified by palpation. In case the implant cannot be palpated or when the presence of the implant is doubtful, see section 4.2 How to insert Nexplanon subsection ‘If the implant is not palpable after insertion’.
When to insert Nexplanon
IMPORTANT: Rule out pregnancy before inserting the implant.
Timing of insertion depends on the woman’s recent contraceptive history, as follows:
No preceding hormonal contraceptive use in the past month
The implant should be inserted between Day 1 (first day of menstrual bleeding) and Day 5 of the menstrual cycle, even if the woman is still bleeding.
If inserted as recommended, back-up contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
Switching hormonal contraceptive method to Nexplanon
Changing from a combined hormonal contraceptive method (combined oral contraceptive (COC), vaginal ring or transdermal patch):
The implant should be inserted preferably on the day after the last active tablet (the last tablet containing the active substances) of the previous combined oral contraceptive or on the day of removal of the vaginal ring or transdermal patch. At the latest, the implant should be inserted on the day following the usual tablet-free, ring-free, patch-free or placebo tablet interval of the previous combined hormonal contraceptive when the next application would have been due. Not all contraceptive methods (transdermal patch, vaginal ring) may be available in all countries.
If inserted as recommended, back-up contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
Changing from a progestagen-only contraceptive method (e.g. progestagen-only pill, injectable, implant, or intrauterine system [IUS]):
As there are several types of progestagen-only methods, the insertion of the implant must be performed as follows:
- Injectable contraceptives: Insert the implant on the day the next injection is due.
- Progestagen-only pill: A woman may switch from the progestagen-only pill to Nexplanon on any day of the month. The implant should be inserted within 24 hours after taking the last tablet.
- Implant/Intrauterine system (IUS): Insert the implant on the same day the previous implant or IUS is removed.
If inserted as recommended, back-up contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
Following abortion or miscarriage
- First trimester: The implant should be inserted within five days following a first trimester abortion or miscarriage.
- Second trimester: Insert the implant between 21 to 28 days following second trimester abortion or miscarriage.
If inserted as recommended, back-up contraception is not necessary. If deviating from the recommended timing of insertion, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
Postpartum
- Not breast-feeding: The implant should be inserted between 21 to 28 days postpartum. If inserted as recommended, back-up contraception is not necessary. If the implant is inserted later than 28 days postpartum, the woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
- Breast-feeding: The implant should be inserted after the fourth postpartum week (see section 4.6). The woman should be advised to use a barrier method until 7 days after insertion. If intercourse has already occurred, pregnancy should be excluded.
How to insert Nexplanon
The basis for successful use and subsequent removal of Nexplanon is a correct and carefully performed subdermal insertion of the implant in the non-dominant arm in accordance with the instructions. Both the HCP and the woman should be able to feel the implant under the woman’s skin after placement.
The implant should be inserted subdermally just under the skin at the inner side of the non-dominant upper arm.
- An implant inserted more deeply than subdermally (deep insertion) may not be palpable and the localisation and/or removal can be difficult (see section 4.2 How to remove Nexplanon and section 4.4).
- If the implant is inserted deeply, neural or vascular damage may occur. Deep or incorrect insertions have been associated with paraesthesia (due to neural damage) and migration of the implant (due to intramuscular or fascial insertion), and in rare cases with intravascular insertion.
Insertion of Nexplanon should be performed under aseptic conditions and only by a qualified HCP who is familiar with the procedure. Insertion of the implant should only be performed with the preloaded applicator.
Insertion Procedure
To help make sure the implant is inserted just under the skin, the HCP should be positioned to see the advancement of the needle by viewing the applicator from the side and not from above the arm. From the side view the insertion site and the movement of the needle just under the skin can be clearly visualised.
For illustrative purposes, Figures depict the left inner arm.
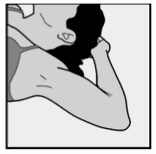
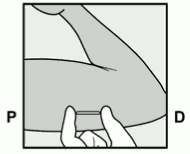
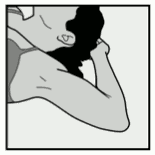
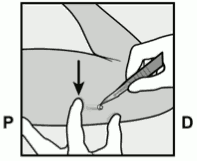
- Have the woman lie on her back on the examination table with her non-dominant arm flexed at the elbow and externally rotated so that her hand is underneath her head (or as close as possible) (Figure 1).
Figure 1:
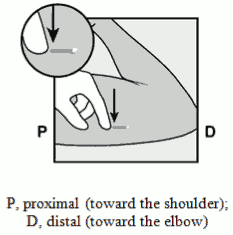
- Identify the insertion site, which is at the inner side of the non-dominant upper arm. The insertion site is overlying the triceps muscle about 8-10 cm (3-4 inches) from the medial epicondyle of the humerus and 3-5 cm (1.25-2 inches) posterior to (below) the sulcus (groove) between the biceps and triceps muscles (Figures 2a, 2b and 2c). This location is intended to avoid the large blood vessels and nerves lying within and surrounding the sulcus. If it is not possible to insert the implant in this location (e.g. in women with thin arms), it should be inserted as far posterior from the sulcus as possible.
- Make two marks with a surgical marker: first, mark the spot where the implant will be inserted, and second, mark a spot at 5 centimetres (2 inches) proximal (toward the shoulder) to the first mark (Figure 2a and 2b). This second mark (guiding mark) will later serve as a direction guide during insertion.
Figue 2a / Figure 2b:
Figure 2c:
After marking the arm, confirm the site is in the correct location on the inner side of the arm.
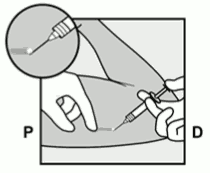
- Clean the skin from the insertion site to the guiding mark with an antiseptic solution.
- Anaesthetise the insertion area (for example, with anaesthetic spray or by injecting 2 ml of 1 % lidocaine just under the skin along the planned insertion tunnel).
- Remove the sterile preloaded disposable Nexplanon applicator carrying the implant from its blister. The applicator should not be used if sterility is in question.
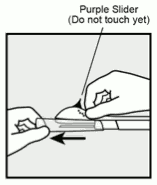
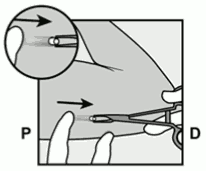
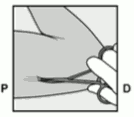
- Hold the applicator just above the needle at the textured surface area. Remove the transparent protection cap by sliding it horizontally in the direction of the arrow away from the needle (Figure 3). If the cap does not come off easily the applicator should not be used. You should see the white coloured implant by looking into the tip of the needle. Do not touch the purple slider until you have fully inserted the needle subdermally, as doing so will retract the needle and prematurely release the implant from the applicator.
- If the purple slider is released prematurely, restart the procedure with a new applicator.
Figure 3:
- With your free hand, stretch the skin around the insertion site towards the elbow (Figure 4).
Figure 4:
- The implant should be inserted subdermally just under the skin (see section 4.4).
To help make sure the implant is inserted just under the skin, you should position yourself to see the advancement of the needle by viewing the applicator from the side and not from above the arm. From the side view you can clearly see the insertion site and the movement of the needle just under the skin (see Figure 6).
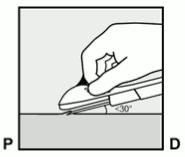
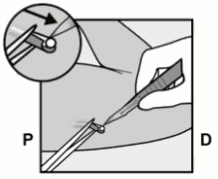
- Puncture the skin with the tip of the needle slightly angled less than 30° (Figure 5a).
Figure 5a:
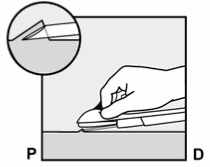
- Insert the needle until the bevel (slanted opening of the tip) is just under the skin (and no further) (Figure 5b). If you inserted the needle deeper than the bevel, withdraw the needle until only the bevel is beneath the skin.
Figure 5b:
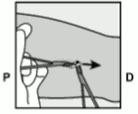
- Lower the applicator to a nearly horizontal position. To facilitate subdermal placement, lift the skin with the needle, while sliding the needle to its full length (Figure 6). You may feel slight resistance but do not exert excessive force. If the needle is not inserted to its full length, the implant will not be inserted properly.
If the needle tip emerges from the skin before needle insertion is complete, the needle should be pulled back and be readjusted to subdermal position to further complete the insertion procedure.
Figure 6:
- Keep the applicator in the same position with the needle inserted to its full length (Figure 7). If needed, you may use your free hand to stabilise the applicator.
Figure 7:
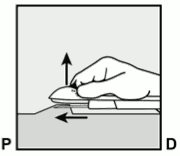
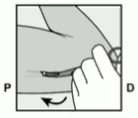
- Unlock the purple slider by pushing it slightly down (Figure 8a). Move the slider fully back until it stops.
Figure 8a:
Do not move the applicator while moving the purple slider (Figure 8b). The implant is now in its final subdermal position, and the needle is locked inside the body of the applicator. The applicator can now be removed (Figure 8c).
Figure 8b:
Figure 8c:
If the applicator is not kept in the same position during this procedure or if the purple slider is not moved fully back until it stops, the implant will not be inserted properly and may protrude from the insertion site.
If the implant is protruding from the insertion site, remove the implant and perform a new procedure at the same insertion site using a new applicator. Do not push the protruding implant back into the incision.
- Apply a small adhesive bandage over the insertion site.
- Always verify the presence of the implant in the woman’s arm immediately after insertion by palpation. By palpating both ends of the implant, you should be able to confirm the presence of the 4 cm rod (Figure 9). See section below “If the implant is not palpable after insertion”.
Figure 9:
- Request that the woman palpate the implant.
- Apply sterile gauze with a pressure bandage to minimise bruising. The woman may remove the pressure bandage in 24 hours and the small adhesive over the insertion site after 3-5 days.
- Complete the Patient Alert Card and give it to the woman to keep. Also, complete the adhesive labels and affix it to the woman’s medical record. If electronic patient records are used, the information on the adhesive label should be recorded.
- The applicator is for single use only and must be adequately disposed of, in accordance with local regulations for the handling of biohazardous waste.
If the implant is not palpable after insertion:
If you cannot palpate the implant or are in doubt of its presence, the implant may not have been inserted or it may have been inserted deeply:
- Check the applicator. The needle should be fully retracted and only the purple tip of the obturator should be visible.
- Use other methods to confirm its presence. Given the radiopaque nature of the implant, suitable methods for localisation are two-dimensional X-ray and X-ray computerised tomography (CT scan). Ultrasound scanning (USS) with a high-frequency linear array transducer (10 MHz or greater) or magnetic resonance imaging (MRI) may be used. In case the implant cannot be found with these imaging methods, it is advised to verify the presence of the implant by measuring the etonogestrel level in a blood sample from the woman. In this case, contact the local representative of the Marketing Authorisation Holder who will provide the appropriate protocol.
- Until you have verified the presence of the implant, the woman must use a non-hormonal contraceptive method.
- Deeply-placed implants should be localised and removed as soon as possible to avoid the potential for distant migration (see section 4.4).
How to remove Nexplanon
Removal of the implant should only be performed under aseptic conditions by an HCP who is familiar with the removal technique. If you are unfamiliar with the removal technique, contact the local representative of the Marketing Authorisation Holder [Merck Sharp & Dohme Limited, Telephone: 01992 467272, medicalinformationuk@merck.com] for further information.
Before initiating the removal procedure, the HCP should assess the location of the implant. Verify the exact location of the implant in the arm by palpation.
If the implant is not palpable, consult the Patient Alert Card or medical record to verify the arm which contains the implant. If the implant cannot be palpated, it may be deeply located or have migrated. Consider that it may lie close to vessels and nerves. Removal of non-palpable implants should only be performed by an HCP experienced in removing deeply placed implants and familiar with localising the implant and the anatomy of the arm. Contact the local representative of the Marketing Authorisation Holder [Merck Sharp & Dohme Limited, Telephone: 01992 467272, medicalinformationuk@merck.com] for further information.
See Section below on “Localisation and removal of a non-palpable implant” if the implant cannot be palpated.
Procedure for removal of an implant that is palpable
For illustrative purposes, Figures depict the left inner arm.
- Have the woman lie on her back on the table. The arm should be positioned with the elbow flexed and the hand underneath the head (or as close as possible). (See Figure 10).
Figure 10:
- Locate the implant by palpation. Push down the end of the implant closest to the shoulder (Figure 11) to stabilise it; a bulge should appear indicating the tip of the implant that is closest to the elbow. If the tip does not pop up, removal of the implant may be difficult and should be performed by providers experienced with removing deeper implants. Contact the local representative of the Marketing Authorisation Holder office [Merck Sharp & Dohme Limited, Telephone: 01992 467272, medicalinformationuk@merck.com] for further information.
Figure 11:
- Mark the distal end (end closest to the elbow), for example, with a surgical marker.
- Clean the site with an antiseptic solution.
- Anesthetise the site, for example, with 0.5 to 1 ml 1% lidocaine where the incision will be made (Figure 12). Be sure to inject the local anesthetic under the implant to keep the implant close to the skin surface. Injection of local anesthetic over the implant can make removal more difficult.
Figure 12:
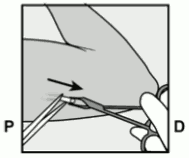
- Push down the end of the implant closest to the shoulder (Figure 13) to stabilise it throughout the procedure. Starting over the tip of the implant closest to the elbow, make a longitudinal (parallel to the implant) incision of 2 mm towards the elbow. Take care not to cut the tip of the implant.
Figure 13:
- The tip of the implant should pop out of the incision. If it does not, gently push the implant towards the incision until the tip is visible. Grasp the implant with forceps and if possible, remove the implant (Figure 14). If needed, gently remove adherent tissue from the tip of the implant using blunt dissection. If the implant tip is not exposed following blunt dissection, make an incision into the tissue sheath and then remove the implant with the forceps (Figures 15 and 16).
Figure 14:
Figure 15:
Figure 16:
- If the tip of the implant does not become visible in the incision, gently insert forceps (preferably curved mosquito forceps, with the tips pointed up) superficially into the incision (Figure 17).
- Gently grasp the implant and then flip the forceps over into your other hand (Figure 18).
- With a second pair of forceps carefully dissect the tissue around the implant and grasp the implant (Figure 19). The implant can then be removed.
- If the implant cannot be grasped, stop the procedure and refer the woman to an HCP experienced with complex removals or contact the local representative of the Marketing Authorisation Holder [Merck Sharp & Dohme Limited, Telephone: 01992 467272, medicalinformationuk@merck.com].
Figure 17:
Figure 18:
Figure 19:
- Confirm that the entire rod, which is 4 cm long, has been removed by measuring its length. There have been reports of broken implants while in the patient’s arm. In some cases, difficult removal of the broken implant has been reported. If a partial implant (less than 4 cm) is removed, the remaining piece should be removed by following the instructions in this section.
- If the woman would like to continue using Nexplanon, a new implant may be inserted immediately after the old implant is removed using the same incision as long as the site is in the correct location (Section 4.2 How to replace Nexplanon).
- After removing the implant, close the incision with a sterile adhesive wound closure.
- Apply sterile gauze with a pressure bandage to minimise bruising. The woman may remove the pressure bandage after 24 hours and the sterile adhesive wound closure after 3-5 days.
Localisation and removal of a non-palpable implant
There have been occasional reports of migration of the implant; usually this involves minor movement relative to the original position (see also section 4.4), but may lead to the implant not being palpable at the location in which it was placed. An implant that has been deeply inserted or has migrated may not be palpable and therefore imaging procedures, as described below, may be required for localisation.
A non-palpable implant should always be located prior to attempting removal. Given the radiopaque nature of the implant, suitable methods for localisation include two-dimensional X-ray and X-ray computer tomography (CT). Ultrasound scanning (USS) with a high-frequency linear array transducer (10 MHz or greater) or magnetic resonance imaging (MRI) may be used. Once the implant has been localised in the arm, the implant should be removed by an HCP experienced in removing deeply placed implants and familiar with the anatomy of the arm. The use of ultrasound guidance during the removal should be considered.
If the implant cannot be found in the arm after comprehensive localisation attempts, consider applying imaging techniques to the chest as extremely rare cases of migration to the pulmonary vasculature have been reported. If the implant is located in the chest, surgical or endovascular procedures may be needed for removal; HCPs familiar with the anatomy of the chest should be consulted.
If at any time these imaging methods fail to locate the implant, etonogestrel blood level determination can be used for verification of the presence of the implant. Please contact the local representative of the Marketing Authorisation Holder for further guidance.
If the implant migrates within the arm, removal may require a minor surgical procedure with a larger incision or a surgical procedure in an operating room. Removal of deeply inserted implants should be conducted with caution in order to help prevent damage to deeper neural or vascular structures in the arm.
Non-palpable and deeply inserted implants should be removed by HCPs familiar with the anatomy of the arm and removal of deeply-inserted implants.
Exploratory surgery without knowledge of the exact location of the implant is strongly discouraged.
Please contact the local representative of the Marketing Authorisation Holder for further guidance.
How to replace Nexplanon
Immediate replacement can be done after removal of the previous implant and is similar to the insertion procedure described in section 4.2 How to insert Nexplanon.
The new implant may be inserted in the same arm, and through the same incision from which the previous implant was removed as long as the site is in the correct location, i.e. 8-10 cm from the medial epicondyle of the humerus and 3-5 cm posterior to (below) the sulcus (see Section 4.2 How to insert Nexplanon). If the same incision is being used to insert a new implant, anaesthetise the insertion site by injecting an anaesthetic (e.g. 2 ml lidocaine (1%)) just under the skin commencing at the removal incision along the ‘insertion canal’ and follow the subsequent steps in the insertion instructions.
Overdose
An implant should always be removed before inserting a new one. There are no data available on overdose with etonogestrel. There have been no reports of serious deleterious effects from an overdose of contraceptives in general.
Shelf life
5 years.
Nexplanon should not be inserted after the expiry date as indicated on the primary package.
Special precautions for storage
This medicinal product does not require any special storage conditions.
Store in the original blister package.
Nature and contents of container
The blister pack contains one implant (4 cm in length and 2 mm in diameter) which is preloaded in the stainless steel needle of a ready-for-use, disposable, sterile applicator. The applicator containing the implant is packed in a blister pack made of transparent polyethyleneterephthalate glycol (PETG) sealed with a lidding made of high density poly ethylene (HDPE). The content of the blister pack is sterile unless the package is damaged or opened.
Pack sizes: Carton box with 1 blister pack, carton box with 5 blister packs.
Not all pack sizes may be marketed.
Special precautions for disposal and other handling
See section 4.2.
The applicator is for single use only.
Any unused medicinal product or waste material should be disposed of in accordance with local requirements.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.