NIASPAN Film coated tablet / Extended-release tablet Ref.[11122] Active ingredients: Nicotinic acid
Source: FDA, National Drug Code (US) Revision Year: 2020
Product description
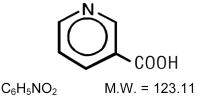
NIASPAN (niacin tablet, film-coated extended-release), contains niacin, which at therapeutic doses is an antihyperlipidemic agent. Niacin (nicotinic acid, or 3-pyridinecarboxylic acid) is a white, crystalline powder, very soluble in water, with the following structural formula:
NIASPAN is an unscored, medium-orange, film-coated tablet for oral administration and is available in three tablet strengths containing 500, 750, and 1000 mg niacin. NIASPAN tablets also contain the inactive ingredients hypromellose, povidone, stearic acid, and polyethylene glycol, and the following coloring agents: FD&C yellow #6/sunset yellow FCF Aluminum Lake, synthetic red and yellow iron oxides, and titanium dioxide.
| Dosage Forms and Strengths |
|---|
|
| How Supplied |
|---|
|
NIASPAN tablets are supplied as unscored, medium-orange, film-coated, capsule-shaped (containing 500 or 750 mg of niacin) or oval shaped (containing 1000 mg of niacin) tablets, in an extended-release formulation. Tablets are supplied in bottles of 90 as shown below.
Manufactured by: AbbVie LTD, Barceloneta, PR 00617 For AbbVie Inc., North Chicago, IL 60064, USA |
Drugs
| Drug | Countries | |
|---|---|---|
| NIASPAN | Hong Kong, Singapore, United States, South Africa |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.