NINLARO Hard capsule Ref.[9136] Active ingredients: Ixazomib
Source: European Medicines Agency (EU) Revision Year: 2019 Publisher: Takeda Pharma A/S, Dybendal Alle 10, 2630, Taastrup, Denmark
Pharmacodynamic properties
Pharmacotherapeutic group: Antineoplastic agents, other antineoplastic agents
ATC code: L01XX50
Mechanism of action
Ixazomib citrate, a prodrug, is a substance that rapidly hydrolyses under physiological conditions to its biologically active form, ixazomib.
Ixazomib is an oral, highly selective and reversible proteasome inhibitor. Ixazomib preferentially binds and inhibits the chymotrypsin-like activity of the beta 5 subunit of the 20S proteasome.
Ixazomib induced apoptosis of several tumour cell types in vitro. Ixazomib demonstrated in vitro cytotoxicity against myeloma cells from patients who had relapsed after multiple prior therapies, including bortezomib, lenalidomide, and dexamethasone. The combination of ixazomib and lenalidomide demonstrated synergistic cytotoxic effects in multiple myeloma cell lines. In vivo, ixazomib demonstrated antitumour activity in various tumour xenograft models, including models of multiple myeloma. In vitro, ixazomib affected cell types found in the bone marrow microenvironment including vascular endothelial cells, osteoclasts and osteoblasts.
Cardiac electrophysiology
Ixazomib did not prolong the QTc interval at clinically relevant exposures based on the results of a pharmacokinetic-pharmacodynamic analysis of data from 245 patients. At the 4 mg dose, mean change from baseline in QTcF was estimated to be 0.07 msec (90% CI; -0.22, 0.36) from the model based analysis. There was no discernible relationship between ixazomib concentration and the RR interval suggesting no clinically meaningful effect of ixazomib on heart rate.
Clinical efficacy and safety
The efficacy and safety of ixazomib in combination with lenalidomide and dexamethasone was evaluated in an international randomised, double-blind, placebo-controlled, multicenter Phase 3 superiority study (C16010) in patients with relapsed and/or refractory multiple myeloma who had received at least one prior therapy. A total of 722 patients (intent-to-treat [ITT] population) were randomised in a 1:1 ratio to receive either the combination of ixazomib, lenalidomide, and dexamethasone (N=360; ixazomib regimen) or placebo, lenalidomide and dexamethasone (N=362; placebo regimen) until disease progression or unacceptable toxicity. Patients enrolled in the trial had multiple myeloma that was refractory, including primary refractory, had relapsed after prior therapy, or had relapsed and was refractory to any prior therapy. Patients that changed therapies prior to disease progression were eligible for enrolment, as well as those with controlled cardiovascular conditions. The Phase 3 study excluded patients who were refractory to lenalidomide or proteasome inhibitors and patients who received more than three prior therapies. For the purposes of this study, refractory disease was defined as disease progression on treatment or progression within 60 days after the last dose of lenalidomide or a proteasome inhibitor. As data are limited in these patients, a careful risk-benefit assessment is recommended before initiating the ixazomib regimen.
Thromboprophylaxis was recommended for all patients in both treatment groups according to the lenalidomide SmPC. Concomitant medicinal products, such as antiemetic, antiviral, and antihistamine medicinal products were given to patients at the physician’s discretion as prophylaxis and/or management of symptoms.
Patients received ixazomib 4 mg or placebo on Days 1, 8, and 15 plus lenalidomide (25 mg) on Days 1 through 21 and dexamethasone (40 mg) on Days 1, 8, 15, and 22 of a 28-day cycle. Patients with renal impairment received a starting dose of lenalidomide according to its SmPC. Treatment continued until disease progression or unacceptable toxicities.
The baseline demographics and disease characteristics were balanced and comparable between the study regimens. The median age was 66 years, range 38-91 years; 58% of patients were older than 65 years. Fifty seven percent of patients were male. Eighty five percent of the population was White, 9% Asian and 2% Black. Ninety three percent of patients had an ECOG performance status of 0-1 and 12% had baseline ISS stage III disease (N=90). Twenty five percent of patients had a creatinine clearance of < 60 mL/min. Twenty three percent of patients had light chain disease and 12% of patients had measurable disease by free light chain assay only. Nineteen percent had high-risk cytogenetic abnormalities (del17, t[4;14], t[14;16]) (N=137), 10% had del(17) (N=69) and 34% had 1q amplification (1q21) (N=247). Patients received one to three prior therapies (median of 1) including prior treatment with bortezomib (69%), carfilzomib (<1%), thalidomide (45%), lenalidomide (12%), melphalan (81%). Fifty seven percent of patients had undergone prior stem cell transplantation. Seventy seven percent of patients relapsed after prior therapies and 11% were refractory to prior therapies. Primary refractory, defined as best response of stable disease or disease progression on all prior therapies, was documented in 6% of patients.
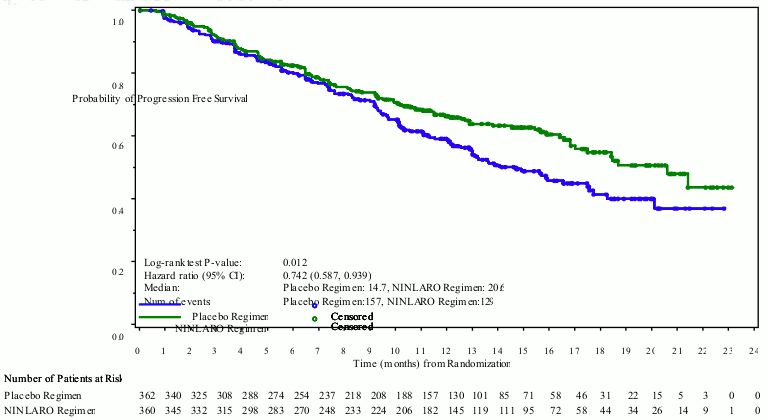
The primary endpoint was progression-free survival (PFS) according to the 2011 International Myeloma Working Group (IMWG) Consensus Uniform Response Criteria as assessed by a blinded independent review committee (IRC) based on central laboratory results. Response was assessed every 4 weeks until disease progression. At the primary analysis (median follow up of 14.7 months and a median of 13 cycles), PFS was statistically significantly different between the treatment arms. PFS results are summarised in Table 4 and Figure 1. The improvement in PFS in the ixazomib regimen was supported by improvements in overall response rate.
Table 4. Progression free survival and response Results in multiple myeloma patients treated with ixazomib or placebo in combination with lenalidomide and dexamethasone (intent-to-treat population):
| Ixazomib + Lenalidomide and Dexamethasone (N=360) | Placebo + Lenalidomide and Dexamethasone (N=362) | |
|---|---|---|
| Progression-Free Survival | ||
| Events, n(%) | 129 (36) | 157 (43) |
| Median (months) | 20.6 | 14.7 |
| p-value* | 0.012 | |
| Hazard Ratio† (95% CI) | 0.74 (0.59, 0.94) | |
| Overall Response Rate‡, n (%) | 282 (78.3) | 259 (71.5) |
| Response Category, n (%) | ||
| Complete Response | 42 (11.7) | 24 (6.6) |
| Very Good Partial Response | 131 (36.4) | 117 (32.3) |
| Partial Response | 109 (30.3) | 118 (32.6) |
| Time to Response, months | ||
| Median | 1.1 | 1.9 |
| Duration of Response§, months | ||
| Median | 20.5 | 15.0 |
* P-value is based on the stratified log-rank test.
† Hazard ratio is based on a stratified Cox’s proportional hazard regression model. A hazard ratio less than 1 indicates an advantage for the ixazomib regimen.
‡ ORR = CR+VGPR+PR
§ Based on responders in the response-evaluable population
Figure 1. Kaplan-Meier plot of progression-free survival in the intent-to-treat population:
A planned interim analysis for overall survival (OS) at a median follow up of 23 months was conducted with 35% of the required number of deaths for final OS analysis in the ITT population; there were 81 deaths in the ixazomib regimen and 90 deaths in the placebo regimen. Median overall survival was not reached in either regimen. At this analysis, estimated median PFS was 20 months in the ixazomib regimen and 15.9 months in the placebo regimen (HR=0.82 [95% CI (0.67, 1.0)]) in the ITT population.
A randomised, double-blind, placebo-controlled Phase 3 study was conducted in China (N=115) with a similar study design and eligibility criteria. Many of the patients enrolled in the study had advanced disease with Durie-Salmon Stage III (69%) at initial diagnosis and a treatment history of receiving at least 2 prior therapies (60%) and being thalidomide refractory (63%). At the primary analysis (median follow up of 8 months and a median of 6 cycles), the median PFS was 6.7 months in the ixazomib regimen compared to 4 months in the placebo regimen (p-value=0.035, HR=0.60). At the final analysis for OS at a median follow up of 19.8 months, OS was improved for patients treated in the ixazomib regimen compared with placebo [p-value=0.0014, HR=0.42, 95% CI: 0.242, 0.726]).
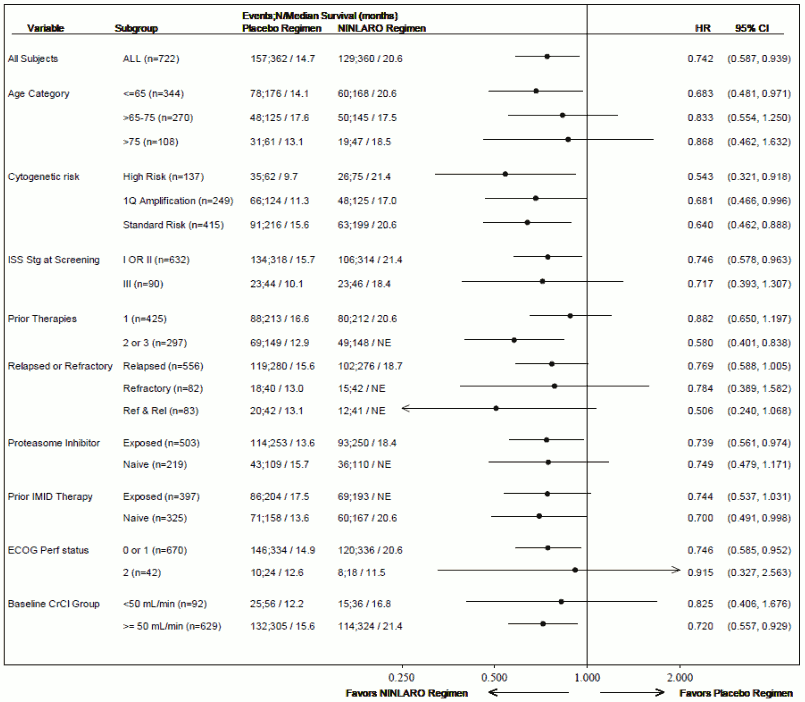
As multiple myeloma is a heterogeneous disease, benefit may vary across subgroups in the Phase 3 study (C16010) (see Figure 2).
Figure 2. Forest plot of progression-free survival in subgroups:
In the Phase 3 study (C16010), 10 patients (5 in each treatment regimen) had severe renal impairment at baseline. Of the 5 patients in the ixazomib regimen, one patient had a confirmed partial response and 3 confirmed stable disease (however 2 were unconfirmed partial response and 1 was an unconfirmed very good partial response). Of the 5 patients in the placebo regimen, 2 had a confirmed very good partial response.
Quality of life as assessed by global health scores (EORTC QLQ-C30 and MY-20) was maintained during treatment and was similar in both treatment regimens in the Phase 3 study (C16010).
Paediatric population
The European Medicines Agency has waived the obligation to submit the results of studies with ixazomib in all subsets of the paediatric population in multiple myeloma (see section 4.2 for information on paediatric use).
Pharmacokinetic properties
Absorption
After oral administration, peak plasma concentrations of ixazomib were achieved at approximately one hour after dosing. The mean absolute oral bioavailability is 58%. Ixazomib AUC increases in a dose proportional manner over a dose range of 0.2-10.6 mg.
Administration with a high-fat meal decreased ixazomib AUC by 28% compared with administration after an overnight fast (see section 4.2).
Distribution
Ixazomib is 99% bound to plasma proteins and distributes into red blood cells with a blood-to-plasma AUC ratio of 10. The steady-state volume of distribution is 543 L.
Biotransformation
After oral administration of a radiolabeled dose, 70% of total drug-related material in plasma was accounted for by ixazomib. Metabolism by multiple CYP enzymes and non-CYP proteins is expected to be the major clearance mechanism for ixazomib. At clinically relevant ixazomib concentrations, in vitro studies using human cDNA-expressed cytochrome P450 isozymes indicate that no specific CYP isozyme predominantly contributes to ixazomib metabolism and non-CYP proteins contribute to overall metabolism. At concentrations exceeding those observed clinically, ixazomib was metabolized by multiple CYP isoforms with estimated relative contributions of 3A4 (42.3%), 1A2 (26.1%), 2B6 (16.0%), 2C8 (6.0%), 2D6 (4.8%), 2C19 (4.8%) and 2C9 (<1%).
Elimination
Ixazomib exhibits a multi-exponential disposition profile. Based on a population PK analysis, systemic clearance (CL) was approximately 1.86 L/hr with inter-individual variability of 44%. The terminal half-life (t½) of ixazomib was 9.5 days. Approximately 2-fold accumulation in AUC was observed with weekly oral dosing on Day 15.
Excretion
After administration of a single oral dose of 14C-ixazomib to 5 patients with advanced cancer, 62% of the administered radioactivity was excreted in urine and 22% in the faeces. Unchanged ixazomib accounted for <3.5% of the administered dose recovered in urine.
Special populations
Hepatic impairment
The PK of ixazomib is similar in patients with normal hepatic function and in patients with mild hepatic impairment (total bilirubin ≤ ULN and AST > ULN or total bilirubin >1-1.5 x ULN and any AST) based on the results of a population PK analysis.
The PK of ixazomib was characterized in patients with normal hepatic function at 4 mg (N=12), moderate hepatic impairment at 2.3 mg (total bilirubin >1.5-3 x ULN, N=13) or severe hepatic impairment at 1.5 mg (total bilirubin >3 x ULN, N=18). Unbound dose-normalized AUC was 27% higher in patients with moderate or severe hepatic impairment as compared to patients with normal hepatic function (see section 4.2).
Renal impairment
The PK of ixazomib is similar in patients with normal renal function and in patients with mild or moderate renal impairment (creatinine clearance ≥30 mL/min) based on the results of a population PK analysis.
The PK of ixazomib was characterized at a dose of 3 mg in patients with normal renal function (creatinine clearance ≥90 mL/min, N=18), severe renal impairment (creatinine clearance <30 mL/min, N=14), or ESRD requiring dialysis (N=6). Unbound AUC was 38% higher in patients with severe renal impairment or ESRD requiring dialysis as compared to patients with normal renal function. Pre- and post-dialyzer concentrations of ixazomib measured during the haemodialysis session were similar, suggesting that ixazomib is not dialyzable (see section 4.2).
Age, gender, race
There was no clinically meaningful effect of age (23-91 years), sex, body surface area (1.2-2.7 m²), or race on the clearance of ixazomib based on the results of a population PK analysis. The mean AUC was 35% higher in Asian patients; however, there was overlap in the AUC of ixazomib across White and Asian patients.
Preclinical safety data
Mutagenicity
Ixazomib was not mutagenic in a bacterial reverse mutation assay (Ames assay) or clastogenic in a bone marrow micronucleus assay in mice. Ixazomib was positive in an in vitro clastogenicity test in human peripheral blood lymphocytes. However, ixazomib was negative in an in vivo comet assay in mice, in which percent tail DNA was assessed in the stomach and liver. Therefore, the weight of evidence indicates that ixazomib is not considered to present a genotoxic risk.
Reproductive and embryo-foetal development
Ixazomib caused embryo-foetal toxicity in pregnant rats and rabbits only at maternally toxic doses and at exposures that were slightly higher than those observed in patients receiving the recommended dose. Studies of fertility and early embryonic development and pre- and post-natal toxicology were not conducted with ixazomib, but evaluation of reproductive tissues was conducted in the general toxicity studies. There were no effects due to ixazomib treatment on male or female reproductive organs in studies up to 6-months duration in rats and up to 9-months duration in dogs.
Animal toxicology and/or pharmacology
In multi-cycle repeated-dose toxicity studies conducted in rats and dogs, the principal target organs included the gastrointestinal tract, lymphoid tissues, and the nervous system. In the 9-month study (10 cycles) in dogs orally administered with a dosing schedule mimicking the clinical regimen (28-day cycle), microscopic neuronal effects were generally minimal in nature and only observed at 0.2 mg/kg (4 mg/m2). The majority of target organ findings demonstrated partial to full recovery following discontinuation of treatment, with the exception of neuronal findings in the lumbar dorsal root ganglion and dorsal column.
Following oral administration, a tissue distribution study in rats revealed that the brain and spinal cord were amongst the tissues with the lowest levels, suggesting that the penetration of ixazomib through the blood-brain barrier appears to be limited. However, the relevance to humans is unknown.
Non-clinical safety pharmacology studies both in vitro (on hERG channels) and in vivo (in telemetered dogs following single oral administration) demonstrated no effects of ixazomib on cardiovascular or respiratory functions at AUC more than 8-fold higher than the clinical value.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.