NITRO-DUR Transdermal patch Ref.[10895] Active ingredients: Glyceryl trinitrate
Source: FDA, National Drug Code (US) Revision Year: 2019
Product description
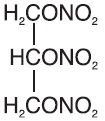
Nitroglycerin is 1,2,3-propanetriol trinitrate, an organic nitrate whose structural formula is:
and whose molecular weight is 227.09. The organic nitrates are vasodilators, active on both arteries and veins.
The NITRO-DUR (nitroglycerin) Transdermal Infusion System is a flat unit designed to provide continuous controlled release of nitroglycerin through intact skin. The rate of release of nitroglycerin is linearly dependent upon the area of the applied system; each cm2 of applied system delivers approximately 0.02 mg of nitroglycerin per hour. Thus, the 5-,10-, 15-, 20-, 30-, and 40-cm2 systems deliver approximately 0.1, 0.2, 0.3, 0.4, 0.6, and 0.8 mg of nitroglycerin per hour, respectively.
The remainder of the nitroglycerin in each system serves as a reservoir and is not delivered in normal use. After 12 hours, for example, each system has delivered approximately 6% of its original content of nitroglycerin.
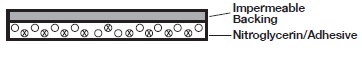
The NITRO-DUR transdermal system contains nitroglycerin in acrylic-based polymer adhesives with a resinous cross-linking agent to provide a continuous source of active ingredient. Each unit is sealed in a paper polyethylene-foil pouch.
Cross section of the system.
| How Supplied | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
* Release rates were formerly described in terms of drug delivered per 24 hours. In these terms, the supplied NITRO-DUR systems would be rated at 2.5 mg/24 hours (0.1 mg/hour), 5 mg/24 hours (0.2 mg/hour), 7.5 mg/24 hours (0.3 mg/hour), 10 mg/24 hours (0.4 mg/hour), and 15 mg/24 hours (0.6 mg/hour). |
Drugs
| Drug | Countries | |
|---|---|---|
| NITRO-DUR | Canada, United States |
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.