NORTHERA Capsule Ref.[10035] Active ingredients: Droxidopa
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
The exact mechanism of action of NORTHERA in the treatment of neurogenic orthostatic hypotension is unknown. NORTHERA is a synthetic amino acid analog that is directly metabolized to norepinephrine by dopa-decarboxylase, which is extensively distributed throughout the body. NORTHERA is believed to exert its pharmacological effects through norepinephrine and not through the parent molecule or other metabolites. Norepinephrine increases blood pressure by inducing peripheral arterial and venous vasoconstriction. NORTHERA in humans induces small and transient rises in plasma norepinephrine.
12.2. Pharmacodynamics
Peak droxidopa plasma concentrations are associated with increases in systolic and diastolic blood pressures. Droxidopa has no clinically significant effect on standing or supine heart rates in patients with autonomic failure.
Cardiac Electrophysiology
No prolongation of the QTc interval was observed with NORTHERA at single oral doses up to 2,000 mg, as shown in a dedicated thorough QT study.
12.3. Pharmacokinetics
Absorption
Peak plasma concentrations (Cmax) of droxidopa were reached by 1 to 4 hours post-dose (mean of approximately 2 hours) in healthy volunteers. High-fat meals have a moderate impact on droxidopa exposure with Cmax and area under the plasma concentration-time curve (AUC) decreasing by 35% and 20%, respectively. The Cmax was delayed by approximately 2 hours with a high-fat meal.
Distribution
Pre-clinical studies suggest that droxidopa can cross the blood brain barrier. Droxidopa exhibits plasma protein binding of 75% at 100 ng/mL and 26% at 10,000 ng/mL. The estimated apparent volume of distribution of droxidopa is about 200 L in humans.
Elimination
The total clearance of droxidopa after oral administration (CL/F) was approximately 400 mL/hr following administration of a single 300 mg dose.
Metabolism
The metabolism of droxidopa is mediated by catecholamine pathway and not through the cytochrome P450 system. Droxidopa is initially converted to methoxylated dihydroxyphenylserine (3-OM-DOPS), a major metabolite, by catechol-O-methyltransferase (COMT), to norepinephrine by DOPA decarboxylase (DDC), or to protocatechualdehyde by DOPS aldolase. After oral dosing in humans, plasma norepinephrine levels peak within 3 to 4 hours but are generally very low (less than 1 ng/mL) and variable with no consistent relationship with dose. The contribution of the metabolites of droxidopa other than norepinephrine to its pharmacological effects is not well understood.
Excretion
The mean elimination half-life of droxidopa is approximately 2.5 hours in humans. The major route of elimination of droxidopa and its metabolites is via the kidneys in both animals and in humans. Studies in animals with radiolabeled drug showed that ~75% of the administered radioactivity was excreted in urine within 24 hours of oral dosing.
Specific Populations
There are no clinically relevant effects of age, body mass index, or sex on the pharmacokinetics of droxidopa. A population pharmacokinetic analysis suggests that hepatic function, assessed by aspartate aminotransferase (AST), alanine aminotransferase (ALT), alkaline phosphatase, and total bilirubin, did not influence the exposure to droxidopa. The controlled clinical trials included patients with mild to moderate renal impairment. No dose adjustments are required in patients with mild to moderate renal impairment.
Drug Interaction Studies
No dedicated drug-drug interaction studies were performed for droxidopa. Patients in the Phase 3 trials with NORTHERA received concomitant levodopa/carbidopa, dopamine agonists, MAO-B inhibitors, COMT inhibitors and other medications used to treat Parkinson’s disease. Carbidopa, a peripheral dopa-decarboxylase inhibitor, could prevent the conversion of NORTHERA to norepinephrine outside of the central nervous system (CNS). Patients taking NORTHERA with L-DOPA/dopa-decarboxylase inhibitor combination drugs had decreased clearance of NORTHERA, an increase in overall exposure (AUC) to droxidopa of approximately 100%, and an increase in overall exposure to 3-OM-DOPS of approximately 50%. However, in clinical trials, it was found that the decreased clearance was not associated with a significant need for a different treatment dose or increases in associated adverse events. Dopamine agonists, amantadine derivatives, and MAO-B inhibitors do not appear to affect NORTHERA clearance, and no dose adjustments are required.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
Long-term studies have been conducted at dosages up to 1,000 mg/kg/day in mice and up to 100 mg/kg/day in rats with no indication of carcinogenic effects. Based on dose per unit body surface area, these two doses correspond to approximately 3 and 0.5 times, respectively, the maximum recommended total daily dose of 1,800 mg in a 60 kg patient. Droxidopa was clastogenic in Chinese hamster ovary cells (chromosome aberration assay), but was not mutagenic in bacteria (Ames assay), and was not clastogenic in a mouse micronucleus assay.
Studies in rats show that droxidopa has no effect on fertility.
13.2. Animal Toxicology and/or Pharmacology
In long-term chronic toxicity studies, rats and mice treated for 52 and 80 weeks, respectively, at doses up to 300 mg/kg/day in rats and 1,000 mg/kg/day in mice had increased incidences of renal and cardiac lesions (rats and mice) and deaths (rats only). The doses at which these effects were not seen represented 0.2 and 0.3 times, in rats and mice, respectively, the maximum recommended total daily dose of 1,800 mg in a 60 kg patient, when based on body surface area.
No signs of toxicity were observed in monkeys or dogs given droxidopa for 13 weeks at doses 32 times (3,000 mg/kg/day) and 37 times (2,000 mg/kg/day), respectively, the maximum human dose.
14. Clinical Studies
14.1 Studies in Neurogenic Orthostatic Hypotension
Clinical studies (described below) examined the efficacy of NORTHERA in the short-term (1 to 2 weeks) and over longer-term periods (8 weeks; 3 months). Studies 301 and 306B showed a treatment effect of NORTHERA at Week 1, but none of the studies demonstrated continued efficacy beyond 2 weeks of treatment.
Study 306B was a multi-center, double-blind, randomized, placebo-controlled, parallel-group study in patients with symptomatic nOH and Parkinson’s disease. Patients entering the study were required to have a decrease of at least 20 mm Hg or 10 mm Hg, respectively, in systolic or diastolic blood pressure, within 3 minutes after standing, as well as symptoms associated with neurogenic orthostatic hypotension. The study had an initial dose titration period that lasted up to 2 weeks in which patients received placebo or 100 to 600 mg of NORTHERA three times daily, followed by an 8-week treatment period.
Efficacy was measured using the OHSA Item #1 score (“dizziness, lightheadedness, feeling faint, and feeling like you might black out”) at Week 1, in patients who had completed titration and 1 week of maintenance therapy.
A total of 171 patients were enrolled, and 147 patients were included in the efficacy analysis. The mean age was 72 years, and patients were mostly Caucasian. During the study, 94% of placebo-treated patients and 88% on NORTHERA were taking dopa-decarboxylase inhibitors; 17% of placebo-treated patients and 26% on NORTHERA were taking fludrocortisone. There were more premature discontinuations in the NORTHERA group (28%) than in the placebo group (20%).
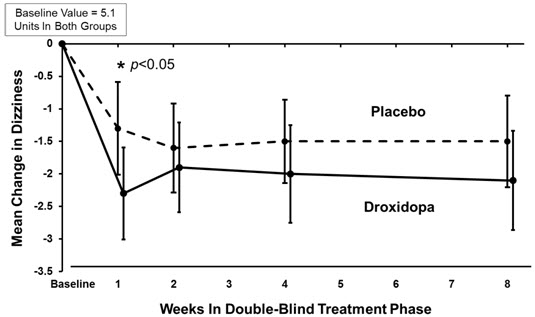
In both groups, the mean baseline dizziness score was 5.1 on an 11-point scale. At Week 1, patients showed a statistically significant mean 0.9 unit decrease in dizziness with NORTHERA versus placebo (P=0.028), but the effect did not persist beyond Week 1. The data at all time points are shown in Figure 1.
Patients receiving NORTHERA also had a greater increase, compared to placebo, in the Week 1 lowest standing systolic blood pressure within 3 minutes after standing (5.6 mm Hg; P=0.032).
Figure 1. Mean Change in OHSA Item 1 Score by Week in Study 306B:
Note: The graph is based on observed data only. The error bars are the 95% confidence interval of the mean change from baseline in OHSA Item 1 scores.
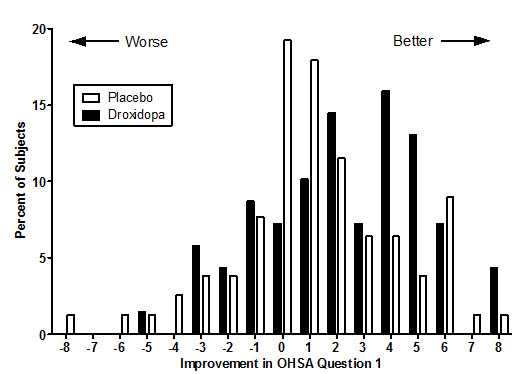
Figure 2. Distribution of Patients by Change in OHSA Item 1, Baseline to Week 1, in Study 306B:
Figure 2 shows the distribution of changes from Baseline to Week 1 in the OHSA Item #1 score. Overall the figure shows that patients treated with NORTHERA improved more than those treated with placebo.
Study 301 was a multicenter, multinational, double-blind, randomized, placebo-controlled, parallel-group study in patients with symptomatic neurogenic orthostatic hypotension. The study included an initial open-label dose titration period, a 7-day washout period, and a randomized double-blind 7-day treatment period. To be eligible for enrollment, patients were required to have a decrease in systolic or diastolic blood pressure of at least 20 or 10 mm Hg, respectively, within 3 minutes after standing. The study was enriched, such that only patients who had been identified as “responders” during the titration period were randomized to NORTHERA or placebo. To be considered a responder, a patient had to demonstrate improvement on the OHSA Item #1 score by at least 1 point, as well as an increase in systolic blood pressure of at least 10 mm Hg post-standing, during the open-label dose titration period. Patients who dropped out during the titration period because of side effects or other reasons were also not included in the double-blind portion of the study.
Patients had a primary diagnosis of Parkinson’s disease (n=60), pure autonomic failure (n=36), or multiple system atrophy (n=26). The mean age was 60 years, and most were Caucasian. 45% of patients were taking dopa-decarboxylase inhibitors, and 29% were taking fludrocortisone.
Efficacy was measured using the Orthostatic Hypotension Questionnaire (OHQ), a patient-reported outcome that measures symptoms of nOH and their impact on the patient’s ability to perform daily activities that require standing and walking. The OHQ includes OHSA Item #1 as one of several components. A statistically significant treatment effect was not demonstrated on OHQ (treatment effect of 0.4 unit, P=0.19).
The mean baseline dizziness score on OHSA Item #1 (“dizziness, lightheadedness, feeling faint, and feeling like you might black out”) was 5.2 units on an 11-point scale. At Week 1 of treatment, patients showed a mean 0.7 unit decrease in dizziness with NORTHERA versus placebo (P=0.06).
Study 302 (n=101) was a placebo-controlled, 2-week randomized withdrawal study of NORTHERA in patients with symptomatic nOH. Study 303 (n=75) was an extension of Studies 301 and 302, where patients received their titrated dose of NORTHERA for 3 months and then entered a 2-week randomized withdrawal phase. Neither study showed a statistically significant difference between treatment arms on its primary endpoint. Considering these data, the effectiveness of NORTHERA beyond 2 weeks is uncertain, and patients should be evaluated periodically to determine whether NORTHERA is continuing to provide a benefit.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.