NOVALGIN Solution for injection Ref.[50517] Active ingredients: Metamizole sodium
Source: Web Search Revision Year: 2017 Publisher: Manufactured by Sanofi Egypt S.A.E under license from Sanofi-Aventis Germany
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: analgesics, other analgesics and antipyretics, pyrazolones
ATC code: N02BB02
Metamizole is a pyrazolone derivative and has analgesic, antipyretic and spasmolytic properties. The mechanism of action is not fully understood. The results of some investigations have shown that metamizole and the main metabolite (4 N-methyl-amino-antipyrine) presumably have both a central and also a peripheral mechanism of action.
5.2. Pharmacokinetic properties
Metamizole is completely hydrolysed after oral administration to the pharmacologically active 4 N methyl-amino-antipyrine (MAA). The bioavailability of MAA is approx. 90% and is slightly higher after oral administration than after parenteral administration. Ingestion with meals has no signicant eect on the kinetics of metamizole.
Its clinical efficacy relies mainly on MAA, to a certain extent also on the metabolite 4 amino-antipyrine (AA). The AUC values for AA represent approx. 25% of the AUC values for MAA. The metabolites 4 N acetyl-amino-antipyrine (AAA) and 4 N-formyl-amino-antipyrine (FAA) appear to be pharmacologically inactive. It should be noted that all metabolites have a non-linear pharmacokinetic action. The clinical significance of this phenomenon is unknown. During short-term treatment, the accumulation of the metabolites is of little signicance. Metamizole crosses the placental barrier. The metabolites of metamizole are excreted in breast milk.
Plasma protein binding for MAA is 58%, for AA 48%, for FAA 18% and for AAA 14%. After intravenous administration the plasma half-life for metamizole is approx. 14 minutes. After intravenous administration approx. 96% of a radioactively labelled dose is recovered in the urine and approx. 6% in the faeces. After a single oral dose 85% of metabolites eliminated in the urine were identified. Of these, 3±1% were MAA, 6±3% AA, 26±8% AAA and 23±4% FAA. Renal clearance of a single oral dose of 1g metamizole was 5±2 for MAA, 38±1 for AA, 61±8 for AAA and 49±5 ml/min for FAA.
The associated plasma half-lives were 2.7±0.5 hours for MAA, 3.7±1.3 hours for AA, 9.5±1.5 hours for AAA and 11.2±1.5 hours for FAA.
Elderly: During the treatment of elderly patients the AUC increases 2 to 3-fold. After oral administration of a single dose in patients with hepatic cirrhosis, the half-lives of MAA and FAA rises approximately 3-fold while the half-lives of AA and AAA do not rise to the same degree. High doses should be avoided in these patients.
Renal impairment: The available data for patients with impaired renal function show a reduced elimination rate for some metabolites (AAA and FAA). High doses should therefore be avoided in these patients.
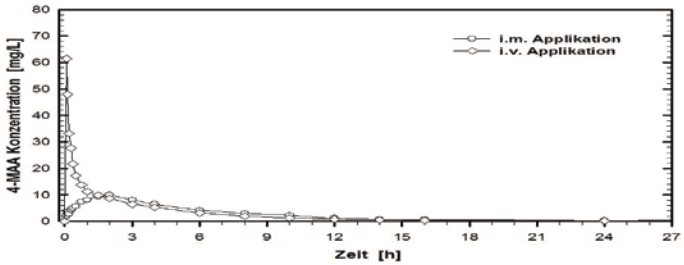
Bioavailability
A bioavailability investigation conducted in 1987 in 12 volunteers with the film-coated tablets compared with a reference product (i.v. administration in 2 minutes) showed the following for 4-MAA:
| i.m. administration (1 g) | i.v. administration (1 g) | |
|---|---|---|
| Peak plasma concentration (Cmax) [mg/l] | 11.4 + 3.12 | 62.1 + 15.9 |
| Time to peak plasma concentration (tmax) [h] | 1.67 + 0.69 | 0.09 + 0.02 |
| Area under the concentration-time curve (AUC) [mg h/l] | 64.1 + 14.8 | 67.8 + 16.1 |
(Values shown as mean and standard deviation)
The absolute bioavailability of the i.m. solution measured from the AUC for 4-MAA-plasma concentrations was 87%.
Fig. 3. Mean plasma curves compared with a reference product in a concentration-time graph:
Key to graph
Konzentration = concentration Applikation = administration
Zeit = time
5.3. Preclinical safety data
Subchronic and chronic toxicity investigations have been performed in various animal species. Rats were given 100 to 900 mg metamizole per kg BW orally for 6 months. At the highest dose (900 mg per kg BW) an increase in reticulocytes and Heinz' bodies were observed after 13 weeks.
Dogs were given metamizole at doses of 30 to 600 mg per kg BW for 6 months.
Dose-related haemolytic anaemia and functional renal and hepatic changes were observed from 300 mg per kg BW.
In-vitro and in-vivo investigations have shown contradictory findings for metamizole in the same test systems.
In long-term investigations in rats no indications of carcinogenic potential were seen. In two out of three long-term investigations in mice, increased liver cell adenomas were reported at high doses.
Embryotoxicity studies in rats and rabbits showed no evidence of teratogenic effects.
Lethal effects on embryos were reported in rabbits from a non-maternotoxic daily dose of 100 mg per kg BW. In rats, fatal embryotoxic eects occurred at doses in the maternotoxic range. Daily doses of more than 100 mg per kg BW in rats led to a prolonged gestation time and impairment of the birth process with increased mortality of dams and young. Fertility tests showed a slightly reduced gravidity rate in the parent generation at a dose of more than 250 mg per kg BW a day. The fertility of the F1 generation was not impaired.
The metabolites of metamizole pass into breast milk. There is no information regarding the effects on the nursing young.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.