NOVOMIX 70 Suspension for injection Ref.[50951] Active ingredients: Insulin aspart
Source: European Medicines Agency (EU) Revision Year: 2020 Publisher: Novo Nordisk A/S, Novo Allé, DK-2880 Bagsværd, Denmark
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Drugs used in diabetes. Insulins and analogues for injection, intermediate- or long-acting combined with fast-acting
ATC code: A10AD05
NovoMix 70 is a biphasic suspension of 70% soluble insulin aspart (rapid-acting human insulin analogue) and 30% protamine-crystallised insulin aspart (intermediate-acting human insulin analogue).
Mechanism of action and pharmacodynamic effects
The blood glucose lowering effect of insulin aspart is due to the facilitated uptake of glucose following binding of insulin to receptors on muscle and fat cells and to the simultaneous inhibition of glucose output from the liver.
NovoMix 70 is a biphasic insulin, which contains 70% soluble insulin aspart. This has a rapid onset of action, thus allowing it to be given closer to a meal (within zero to 10 minutes of the meal) when compared to soluble human insulin. The crystalline phase (30%) consists of protamine-crystallised insulin aspart, which has an activity profile similar to that of human NPH insulin.
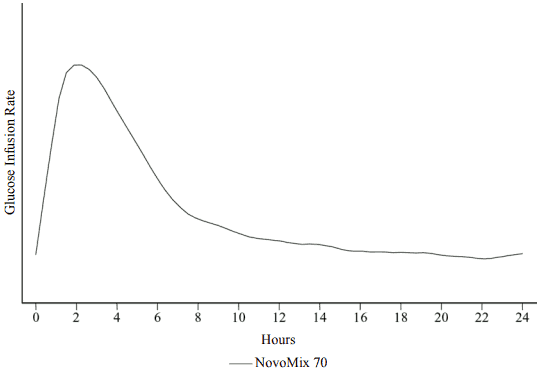
When NovoMix 70 is injected subcutaneously, the onset of action will occur within 10 to 20 minutes of injection. The maximum effect is exerted between 1 and 4 hours after injection. The duration of action is 14 to 24 hours (Figure 1).
Figure 1. Activity Profile for NovoMix 70 in Healthy Caucasian Subjects:
Insulin aspart is equipotent to human insulin on a molar basis.
5.2. Pharmacokinetic properties
Absorption, distribution and elimination
In insulin aspart, substitution of amino acid proline with aspartic acid at position B28 reduces the tendency to form hexamers as observed with soluble human insulin. The insulin aspart in the soluble phase of NovoMix 70 comprises 70% of the total insulin; this is absorbed more rapidly from the subcutaneous layer than the soluble insulin component of biphasic human insulin. The remaining 30% is in crystalline form as protamine-crystallised insulin aspart; this has a prolonged absorption profile similar to human NPH insulin.
In healthy volunteers, a mean maximum serum concentration of 645 ± 185 pmol/l was reached about 60 minutes after a subcutaneous dose of 0.30 unit/kg body weight. In type 2 patients with diabetes, the maximum concentration was reached about 75 minutes after dosing. In type 1 patients with diabetes a mean maximum serum concentration of 721 ± 184 pmol/l was reached about 60 minutes after a subcutaneous dose of 0.30 unit/kg body weight.
Special populations
The pharmacokinetics of NovoMix 70 have not been investigated in paediatrics, elderly patients or in patients with renal or hepatic impairment.
5.3. Preclinical safety data
Non-clinical data reveal no special hazard for humans based on conventional studies of safety pharmacology, repeated dose toxicity, genotoxicity and toxicity to reproduction and development.
In in vitro tests, including binding to insulin and IGF-1 receptor sites and effects on cell growth, insulin aspart behaved in a manner that closely resembled human insulin. Studies also demonstrate that the dissociation of binding to the insulin receptor of insulin aspart is equivalent to human insulin.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.