OKEDI Powder and solvent for suspension for injection Ref.[49627] Active ingredients: Risperidone
Source: European Medicines Agency (EU) Revision Year: 2022 Publisher: Laboratorios Farmacéuticos Rovi, S.A., Julián Camarillo, 35, 28037 Madrid., Spain
5.1. Pharmacodynamic properties
Pharmacotherapeutic group: Psycholeptics, other antipsychotics
ATC code: N05AX08
Mechanism of action
Risperidone is a selective monoaminergic antagonist with unique properties. It has a high affinity for serotoninergic 5-HT2 and dopaminergic D2 receptors. Risperidone binds also to alpha1-adrenergic receptors, and, with lower affinity, to H1-histaminergic and alpha2-adrenergic receptors. Risperidone has no affinity for cholinergic receptors. Although risperidone is a potent D2 antagonist, which is considered to improve the positive symptoms of schizophrenia, it causes less depression of motor activity and induction of catalepsy than classical antipsychotics. Balanced central serotonin and dopamine antagonism may reduce extrapyramidal side effect liability and extend the therapeutic activity to the negative and affective symptoms of schizophrenia.
Pharmacodynamic effects
Clinical efficacy
The efficacy of OKEDI (75 mg and 100 mg) in the treatment of schizophrenia in adults was established in one Phase 3, multicentre, randomised, DB, placebo-controlled, parallel groups study. The study admitted patients with an acute exacerbation or relapse of schizophrenia (DSM-5 criteria), who had a baseline Positive and Negative Syndrome Scale (PANSS) score of 80-120. At the screening visit, all risperidone naïve patients received 2 mg/day oral risperidone for 3 days to ensure a lack of hypersensitivity reactions before the trial. Patients with previous history of being treated with risperidone did not receive oral risperidone at the screening and started directly with OKEDI (75 mg or 100 mg) or placebo after randomization. Four hundred and thirty-eight (438) patients were randomised to receive 3 intramuscular doses of OKEDI (75 mg or 100 mg) or placebo every 28 days. The mean age of patients was 42.0 (SD: 11.02) years. No patients <18 years or >65 years were included. Demographic and other baseline characteristics were similar in each treatment group. No supplemental oral risperidone was permitted during the study.
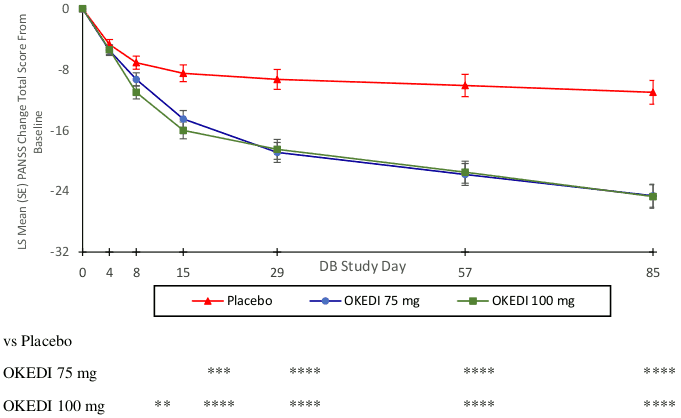
The primary endpoint was the change in PANSS total score from baseline to end of study (Day 85). Both OKEDI 75 and 100 mg doses demonstrated a statistically significant improvement compared with placebo based on the primary endpoint (Table 1 and Figure 1). These results support efficacy across the entire duration of treatment and improvement in PANSS and was observed as early as day 4 with significant separation from placebo in the 100 mg and 75 mg groups by day 8 and 15, respectively. Similar to the PANSS Total Score, the three PANSS positive, negative and general psychopathological subscale scores also showed an improvement (decrease) from baseline over time.
Table 1. Mean change in PANSS and CGI-S total score from baseline to the end of study (day 85) (mITT Population):
| Placebo N=132 | OKEDI 75 mg N=129 | OKEDI 100 mg N=129 | |
|---|---|---|---|
| PANSS total scorea | |||
| Mean baseline score (SD) | 96.4 (7.21) | 96.3 (8.47) | 96.1 (8.42) |
| LS Mean Change, 95% CIa | -11.0, -14.1 to -8.0 | -24.6, -27.5 to -21.6 | -24.7, -27.7 to -21.6 |
| Treatment Difference, 95% CIb | -13.0, -17.3 to -8.8 | -13.3, -17.6 to -8.9 | |
| P-value | <0.0001 | <0.0001 | |
| CGI-S total scorec | |||
| Mean baseline score (SD) | 4.9 (0.52) | 5.0 (0.65) | 4.9 (0.48) |
| LS Mean Change, 95% CIa | -0.6, -0.8 to -0.4 | 1.3, -1.5 to -1.2 | -1.3, -1.5 to -1.2 |
| Treatment Difference, 95% CIb | -0.7, -1.0 to -0.5 | -0.7, -1.0 to -0.5 | |
| P-value | <0.0001 | <0.0001 | |
a Data were analyzed using a mixed model repeated measures (MMRM) approach.
b Difference (OKEDI minus placebo) in least squares mean change from baseline adjusted by Lawrence and Hung method.
c The Clinical Global Impression – Severity (CGI-S) score asks the clinician one question: “Considering your total clinical experience with this particular population, how mentally ill is the patient at this time?” which is rated on the following seven-point scale: 1=normal, not at all ill; 2=borderline mentally ill; 3=mildly ill; 4=moderately ill; 5=markedly ill; 6=severely ill; 7=among the most extremely ill patients.
Figure 1. PANSS Total Score Change from Baseline at Each Time Point in DB Phase (mITT Population):
** p<0.01, *** p<0.001, **** p<0.0001.
The key secondary efficacy endpoint was defined as the mean change from baseline at Day 85 on the Clinical Global Impression – Severity (CGI-S) score. Both OKEDI treatment groups demonstrated statistically significantly better CGI-S scores versus placebo from day 8 onwards (-0.4 (0.05) and -0.6 (0.05) score reduction from baseline for 75 mg and 100 mg, respectively).
Overall Response (PANSS total score reduction >30% and/or CGI-I of 2 “much improved” or 1 “very much improved”) rate at endpoint for OKEDI was 56% and statistically significant from Day 8 and 15 onwards for both doses in comparison to placebo.
The long-term (12 months) efficacy of OKEDI was evaluated in an open-label extension of the main study in 215 patients with schizophrenia. The extension study was open to enrolment for patients from the DB phase (rollover patients) and stable patients not previously enrolled in the study (de novo patients). The de novo patients were switched from oral risperidone to OKEDI 75 mg or 100 mg. Efficacy was maintained over time with a relapse rate of 10.7% (95% CI, 6.9% to 15.6%) and a remittance rate of 61.0% (95% CI, 53.7% to 68.4%).
5.2. Pharmacokinetic properties
Risperidone is metabolised to 9-hydroxy-risperidone, which has a similar pharmacological activity to risperidone (see Biotransformation and Elimination).
Absorption
OKEDI contains risperidone in a suspension delivery system that shows a combined absorption process. Following intramuscular injection, a small amount of the drug is immediately released at the moment of the injection that provides immediate plasma levels. After a first peak concentration, mean plasma concentrations decrease sustainedly through Day 14 and then increased again to reach a second peak between approximately Day 21 and Day 24. Following the second peak, plasma concentrations decreased gradually over time. The suspension forms a depot that provides sustained therapeutic plasma concentrations that are maintained over the 28-day interval.
After single IM injection of OKEDI 75 and 100 mg, mean active moiety concentrations of 13 ± 9 and 29 ± 13 ng/mL respectively are achieved at 2 hours after administration. Active moiety plasma concentrations of 17 ± 8 and 21 ± 17 ng/mL respectively one month after administration, and in most of the patients the drug is completely eliminated 75 days after administration, with active moiety values lower than 1 ng/ml.
The mean trough plasma concentrations (Ctrough). and mean maximum peak plasma concentrations (Cmax) of active moiety following repeated intramuscular injections with OKEDI are shown in Table 2.
Table 2: Ctrough and Cmax of active moiety following repeated intramuscular injections with OKEDI:
| Dose | Ctrough (SD) ng/mL | Cmax (SD) ng/mL |
|---|---|---|
| 75 mga | 17.6 | 35.9 |
| 100 mgb | 28.9 (13.7) | 69.7 (27.8) |
a Summary simulated estimates pharmacokinetic (PK) variables following the 3rd dose of OKEDI 75 mg using population (pop) PK model
b Summary statistics PK variables following the 4th dose of OKEDI 100 mg from multiple dose clinical trial
SD: standard deviation
Steady state concentrations for the typical subject were attained following the first dose.
The average exposure at steady state was similar for both deltoid and gluteal injection sites.
Distribution
Risperidone is rapidly distributed. The volume of distribution is 1-2 l/kg. In plasma, risperidone is bound to albumin and alpha1-acid glycoprotein. The plasma protein binding of risperidone is 90% that of 9-hydroxy-risperidone is 77%.
Biotransformation and elimination
Risperidone is metabolised by CYP2D6 to 9-hydroxy-risperidone, which has a similar pharmacological activity as risperidone. Risperidone plus 9-hydroxy-risperidone form the active moiety. CYP2D6 is subject to genetic polymorphism. Extensive CYP2D6 metabolisers convert risperidone rapidly into 9-hydroxy-risperidone, whereas poor CYP2D6 metabolisers convert it much more slowly. Although extensive metabolisers have lower risperidone and higher 9-hydroxy-risperidone concentrations than poor metabolisers, the pharmacokinetics of risperidone and 9-hydroxy-risperidone combined (i.e., the active moiety), after single and multiple doses, are similar in extensive and poor metabolisers of CYP2D6.
Another metabolic pathway of risperidone is N-dealkylation. In vitro studies in human liver microsomes showed that risperidone at clinically relevant concentration does not substantially inhibit the metabolism of medicines metabolised by cytochrome P450 isozymes, including CYP1A2, CYP2A6, CYP2C8/9/19, CYP2D6, CYP2E1, CYP3A4, and CYP3A5. One week after administration, 70% of the dose is excreted in the urine and 14% in the faeces. In urine, risperidone plus 9-hydroxy-risperidone represent 35-45% of the dose. The remainder is inactive metabolites. After oral administration to psychotic patients, risperidone is eliminated with a half-life of about 3 hours. The elimination half-life of 9-hydroxy-risperidone and of the active moiety is 24 hours.
The active moiety is eliminated within 75 days after OKEDI administration, with active moiety values lower than 1 ng/mL in most of the patients.
OKEDI injection versus oral risperidone
Initial plasma levels with OKEDI were within the exposure range observed with 3-4 mg of oral risperidone. Steady state exposure after OKEDI 100 mg compared to 4 mg oral risperidone was 39% higher for AUC and 32% for Cmax and was similar for Cmin. Simulations based on population pharmacokinetic modelling show that OKEDI 75 mg exposure is similar to 3 mg oral risperidone at steady state.
When switching from oral risperidone to OKEDI, the predicted exposure of the active moiety is in a similar range, including peak concentrations.
Linearity/non-linearity
OKEDI has been found to exhibit linear and dose-proportional pharmacokinetics at doses of 75 and 100 mg.
Elderly
OKEDI has not been systematically studied in elderly patients (see section 4.2).
Renal impairment
OKEDI has not been systematically studied in patients with renal impairment. Patients with mild renal impairment (creatinine clearance 60 to 89 mL/min) that received OKEDI administration showed similar active moiety exposure than patients with normal renal function.
No data is available in moderate renal disease or severe renal disease.
Hepatic impairment
OKEDI has not been systematically studied in patients with hepatic impairment.
Body mass index (BMI)
Population pharmacokinetic simulations have shown potential increases in plasma concentrations of OKEDI in obese or morbid obese females in comparison with normal weight patients with insignificant clinical impact.
Gender, race and smoking habits
A pop PK analysis revealed no apparent effect of gender, race or smoking habits on the pharmacokinetics of risperidone or the active moiety.
5.3. Preclinical safety data
In vitro and in vivo, animal models show that at high doses risperidone may cause QT interval prolongation, which has been associated with a theoretically increased risk of Torsade de Pointes in patients.
In (sub)chronic oral toxicity studies, in which dosing was started in sexually immature rats and dogs, dose-dependent effects were present in male and female genital tract and mammary gland. These effects were related to the increased serum prolactin levels, resulting from the dopamine D2 receptor blocking activity of risperidone. In addition, tissue culture studies suggest that cell growth in human breast tumours may be stimulated by prolactin.
The major effects of treatment with OKEDI observed following chronic (12 months of intramuscular administration) toxicity studies in dogs and rabbits were in accordance with the findings following oral distribution of risperidone in rats and dogs, and related to the pharmacological effects of risperidone.
Local alterations, nodules, at the injection site in 12-cycle toxicity studies in dogs and rabbits were observed after intramuscularly administration of OKEDI. They consisted of muscular foreign body granulomatous inflammation attributed to natural body response to the presence of a foreign substance. Other local alterations observed in rabbits at 15 mg/kg (risperidone) were related to Dimethyl sulfoxide (DMSO) content. These all alterations were strictly local and there was evidence of reversibility. In dogs, transient pain associated to DMSO content was observed immediately after administration.
There was no evidence of genotoxic potential for either risperidone or for OKEDI.
In oral carcinogenicity studies of risperidone in rats and mice, increases in pituitary gland adenomas (mouse), endocrine pancreas adenomas (rat), and mammary gland adenomas (both species) were seen. These tumours can be related to prolonged dopamine D2 antagonism and hyperprolactinaemia. The relevance of these tumour findings in rodents in terms of human risk is unknown.
Risperidone was not teratogenic in rat and rabbit. In rat reproduction studies with risperidone, adverse effects were seen on mating behaviour of the parents, and on the birth weight and survival of the offspring. In rats, intrauterine exposure to risperidone was associated with cognitive deficits in adulthood. Other dopamine antagonists, when administered to pregnant animals, have caused negative effects on learning and motor development in the offspring.
In a toxicity study in juvenile rats, increased pup mortality and a delay in physical development was observed. In a 40-week study with juvenile dogs, sexual maturation was delayed. Based on area under the curve (AUC), long bone growth was not affected in dogs at 3.6-times the maximum human exposure in adolescents (1.5 mg/day); while effects on long bones and sexual maturation were observed at 15 times the maximum human exposure in adolescents.
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.