OMEGAVEN Emulsion for injection Ref.[27455] Active ingredients: Omega-3-acid ethyl esters
Source: FDA, National Drug Code (US) Revision Year: 2020
12.1. Mechanism of Action
Omegaven provides a biologically utilizable source of calories and essential fatty acids.
Fatty acids serve as an important substrate for energy production. The most common mechanism of action for energy production derived from fatty acid metabolism is beta oxidation. Fatty acids are also important for membrane structure and function, as precursors for bioactive molecules (such as prostaglandins), and as regulators of gene expression.
12.3. Pharmacokinetics
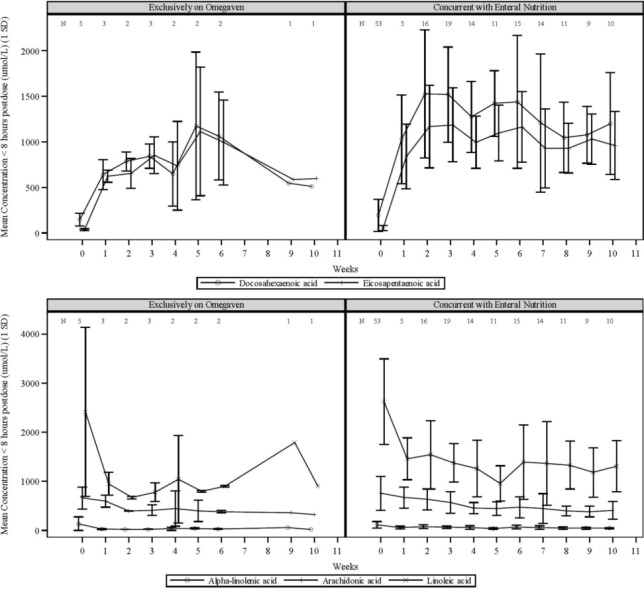
The plasma concentrations of EPA and DHA, the major fatty acids in Omegaven, as well as linoleic acid and alpha-linolenic acid (essential fatty acids) were measured along with the markers of essential fatty acid status in 58 pediatric patients with PNAC after an intravenous infusion of 1 mg/kg/day of Omegaven over 10 weeks. Five patients received Omegaven as the exclusive lipid source, and all others received concurrent enteral or oral nutrition.
Figure 1. Mean Plasma Concentrations of Fatty Acids Over 10 Weeks of Omegaven Infusion in Pediatric Patients with PNAC:
Error bars represent ± 1 standard deviation (SD).
Numbers at the top of plots represent the number of patients at each time point
If more than one value was available for a patient at any given time point, the average was used.
13.1. Carcinogenesis, Mutagenesis, Impairment of Fertility
No studies have been performed with fish oil triglycerides to evaluate the carcinogenic potential or its effect on fertility.
Fish oil triglycerides was negative in the bacterial mutagenicity test with Salmonella typhimurium and the hypoxanthine phosphoribosyl transferase (HPRT) gene mutation assay in Chinese hamster V79 cells. Fish oil triglycerides was not clastogenic in cultured human peripheral lymphocytes or in a rat bone marrow cytogenetic study.
14. Clinical Studies
The efficacy of Omegaven was evaluated in two open-label single-center clinical trials (Study 1, NCT00910104, and Study 2, NCT00738101) in pediatric patients with PNAC (defined as direct or conjugated bilirubin [DBil] equal to or greater than 2 mg/dL) who required PN for at least 14 days. Although Study 1 and Study 2 were not adequately designed to demonstrate noninferiority or superiority of Omegaven to the soybean oil-based lipid emulsion comparator, the data from these studies support Omegaven as a source of calories in pediatric patients with PNAC. Nutritional efficacy was assessed by biomarkers of lipid metabolism, growth indices (body weight, length/height and head circumference), and/or mean changes in fatty acid parameters.
Both trials prospectively enrolled Omegaven-treated patients (maximum dose of 1 g/kg/day) and used historical control patients who received a soybean oil-based lipid emulsion (maximum dose of 3 g/kg/day) as a comparator. Patients were expected to require PN, which also included dextrose, amino acids, vitamins and trace elements, for at least 30 days (Study 1) or 14 days (Study 2), had PNAC, and had received standard therapies to prevent progression of liver disease. Study 1 enrolled patients less than 2 years of age and Study 2 enrolled patients less than 5 years of age. Patients with another cause of chronic liver disease (in the absence of intestinal failure) were excluded. Patients with an international normalized ratio (INR) greater than 2 and patients with portal vein thrombosis or reversal of portal flow by abdominal ultrasound were also excluded.
For the efficacy analyses of Studies 1 and 2, Omegaven-treated patients were pair-matched in a 2:1 manner to historical control patients primarily based on DBil levels and postmenstrual age at baseline. There were 123 patients (82 Omegaven; 41 historical control) in this population, 78 (52; 26) were from Study 1, and 45 (30; 15) were from Study 2. A summary of concurrent enteral/oral nutrition intake for each study is provided in Table 2.
Table 2. Summary of Median Enteral or Oral Intakes in Pediatric Patients with PNAC in Study 1 and Study 2:
| Parameter | Study 1 | Study 2 | ||
|---|---|---|---|---|
| Omegaven (n=50)a | Historical Control (n=26) | Omegaven (n=30) | Historical Control (n=15) | |
| Number of patients who received concurrent enteral or oral nutrition | 44 (88%) | 26 (100%) | 24 (80%) | 14 (93%) |
| Percentage of total calories provided enterally or orally, median (Min – Max) | 24% (1% – 53%) | 25% (0.4% – 68%) | 21% (1% – 75%) | 12% (3% – 40%) |
a. Two Omegaven-treated patients in Study 1 did not have data regarding enteral or oral intakes.
In the combined efficacy analysis population from Study 1 and Study 2, median chronological age was 9 weeks (range: 3 to 42 weeks) in the Omegaven group and 7 weeks (range: 0 to 41 weeks) in the historical control group. The majority of these patients were preterm infants at birth (90% Omegaven; 83% historical control), with gestational age categories as follows: extremely preterm (31%; 20%); very preterm (20%; 24%); moderate or late preterm (40%; 39%). A majority of patients were also considered to have low, very-low, or extremely-low birth weights (76%; 82%), with birth weight categories as follows: extremely-low birth weight (34%; 24%); very-low birth weight (17%; 21%); low birth weight (25%; 37%).
The efficacy analysis population had more males (51%; 59%) than females, and the majority of patients were White (60%; 66%).
At baseline, the median age-adjusted body weight (Z-score) was -1.3 for the Omegaven group and -1.1 for the historical control group; 27% and 28% were low-for-age in body weight, 43% and 40% were low-for-age in body height/length, and 25% and 15% were low-for-age in head circumference for the Omegaven and historical control groups, respectively (low-for-age corresponded to Z-scores less than or equal to -1.9 for each growth parameter). In the efficacy analysis population, baseline median DBil, AST, and ALT levels were 3.8 mg/dL, 101 U/L, and 67 U/L, respectively, for the Omegaven group; and 3.8 mg/dL, 115 U/L, and 52 U/L, respectively, for the historical control group.
The median (range) of the duration of treatment was 2.7 months (5 days to 8 years) for the Omegaven group and 3.6 months (16 days to 2 years) for the historical control group.
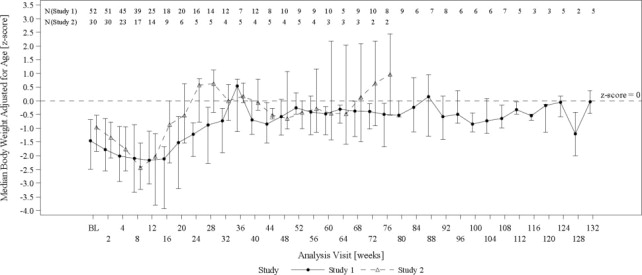
The changes in median age-adjusted body weight (Z-scores) over time for Omegaven-treated patients (Figure 2) appeared similar to those for historical control patients. In both the Omegaven and historical control groups, there was an initial decline in all growth parameters (weight, height/length, head circumference) over the initial weeks of treatment, followed by catch-up growth and more age-appropriate values through the remainder of the study. By comparing the Omegaven study data to age-standardized Fenton and World Health Organization (WHO) growth charts to assess age appropriate growth in patients with PNAC, patients treated with Omegaven as their exclusive lipid source also achieved age-appropriate growth.
Figure 2. Median Age-Adjusted Body Weight (Z-scores) Over Time in Omegaven-Treated Pediatric Patients with PNAC in Study 1 and Study 2*:
BL = baseline
Error bars represent interquartile ranges.
* Data from pair-matched Omegaven patients were truncated at week 132. Median values are only shown for visits with data from at least 2 patients at a particular visit.
In the combined analysis from Study 1 and Study 2, the number of Omegaven and historical control patients who achieved full enteral feeding by the end of the study was 52 (63%) patients and 24 (59%) patients, respectively. The median time to full enteral feeding was approximately 15 weeks for both groups.
At the end of the studies, the median DBil level for Omegaven-treated patients was 0.60 mg/dL (interquartile range: 0.1 to 2.8 mg/dL). The Kaplan-Meier estimate of the median time for DBil values to return to less than 2.0 mg/dL was approximately 5.7 weeks [see Dosage and Administration (2.3), Adverse Reactions (6.1)].
© All content on this website, including data entry, data processing, decision support tools, "RxReasoner" logo and graphics, is the intellectual property of RxReasoner and is protected by copyright laws. Unauthorized reproduction or distribution of any part of this content without explicit written permission from RxReasoner is strictly prohibited. Any third-party content used on this site is acknowledged and utilized under fair use principles.